Abstract
Rational: Patients under regular dialysis can also present alterations in the cardiovascular, musculoskeletal, and metabolic systems. Objectives: The aim of this study is to compare the effects of strength and aerobic exercises performed during hemodialysis (HD) in individuals with chronic renal disease. Materials and Methods: Randomized clinical trial. It was developed as a program of exercises three times a week, in the first 2 h of HD for 8 weeks. The patients were divided into three groups: control (Group 1, n: 11), strength (Group 2, n: 11), and aerobic (Group 3, n: 10). G1 has not developed any type of physical training; G2 utilized a training load of 40% of one repetition maximum (1RM) with anklets, and developed three series of 15 repetitions. G3 pedaled seated in the dialysis seat, during 20 min, in an ergometric bicycle, with intensity regulated by the perceived effort scale. Before and after 8 weeks, the following variables were evaluated: respiratory muscular strength, pulmonary function, functional capacity, blood biochemistry, and quality of life. Main Findings: In the pre- and post-training comparison, there was statistically significant improvement (p < 0.05) in the maximal inspiratory pressure (MIP), number of steps achieved (NSA), and quality of life (QoL) in the trained groups, as compared to the non-exercised group (G1). Conclusions: The strength and aerobic exercises developed during HD can improve the respiratory muscular strength, functional performance, and quality of life, when compared to individuals presenting the disease who have not developed any type of physical training.
INTRODUCTION
The advanced chronic renal failure (CRF) characterizes itself by irreversible renal injury, interfering directly in glomerular filtration. In this stage, substitutive renal therapy (SRT) with dialytic treatment and/or the development of renal transplantation are employed.Citation1,Citation2
The main risk factors for development of CRF are systemic arterial hypertension (SAH), diabetes mellitus, and glomerulonephritis. Besides these factors, inflammatory processes, oxidative stress, endothelial dysfunction, uremia, and familial antecedents can also contribute to the development of renal disease.Citation3
With the progress in course in dialytic treatment, the life expectation of these patients is increasing significantly; however, factors like disease stage, physical activity level, and cardiovascular disease contribute to the decrease in the quality of life of individuals with chronic renal disease.Citation4,Citation5 Besides, patients under regular dialysis can also present alterations in the cardiovascular, musculoskeletal, and metabolic systems, and such alterations can compromise 40–50% of their exercise capacity and peripheral muscular strength.Citation6–Citation8
It is suggested that the intolerance to exercise commonly presented by CRF patients results from the decrease in physical aptitude, which is caused by their low capacity of oxygen transportation and decreased extraction of oxygen from peripheral skeletal musculature.Citation5 The circulating toxins, excess of body liquid, electrolytic disturbances, nutritional alterations, as well as the inactiveness itself and the presence of inflammatory processes all contribute together, in direct or indirect manner, for the appearance of these situations, thus decreasing the patients’ survival.Citation9,Citation10
Studies demonstrate that exercises developed with patients subjected to hemodialysis (HD), in the first 2 h of this procedure, can present a gain in “aerobic capacity,” “muscular strength,” an “increase in dialysis efficiency,” as well as an improvement in their “toxin clearance” and “quality of life.” However, there is no consensus in the literature about which exercise program would be more indicated to be developed during HD.Citation11–Citation13
The routine prescription of physical exercises during HD is still uncommon; so, it is necessary to amplify the understanding about their effects in this group of individuals. After the clarification of such variables, the physical activity could be developed in a safer manner, and their results could be beneficial for this population.Citation5,Citation11,Citation14–Citation17
The purpose of this study was to compare two types of physical exercises developed during HD (strength vs. aerobic) and their influence on the muscular strength, functional capacity, pulmonary function, and quality of life.
METHODS
Patients
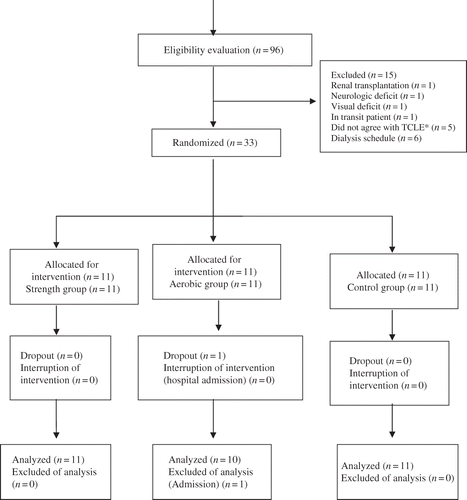
Thirty-two patients participated in this study, according to the randomization procedure illustrated in flowchart.
This was a randomized clinical trial type study, developed with 32 outpatients of the dialysis unit of Nefroclínica, in Foz do Iguaçu (state of Paraná, Brazil).
The entire project was approved by the Institutional Review Board/Independent Ethics Committee of IPA, in Porto Alegre (state of Rio Grande do Sul), under the protocol 279/2009; and the signature of both, the Investigators Agreement by the responsible investigators and the Informed Consent Form by the volunteer participants, was required.
The inclusion criteria for this study were as follows: patients being regularly subjected to HD, three times a week; the gender being irrespective; being aged between 18 and 75 years; and not practicing any physical activity.
The exclusion criteria were uncontrolled arterial hypertension, ischemic cardiopathy, amputation, deep vein thrombosis, excessive pallor, severe dyspnea, femoral fistula, arrhythmias, precordial pain, orthopedic or neurological compromising, and cognitive alterations affecting their participation in the proposed protocol.
The clinical and anthropometric characteristics of participants were verified in a previous evaluation and by the analysis of medical history, in which were collected also the clinical diagnoses indicating renal disease, age, gender, body mass index (BMI), time of disease and of dialysis, as well as smoking habits. The variables of respiratory muscular strength, pulmonary function, tolerance to submaximal exercise, laboratory examinations, and quality of life were measured before and after 8 weeks of exercise programs.
Procedures
As respiratory muscular strength markers, the maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) were evaluated through an analogical manovacuometer (Globalmed ± 300 cmH2O, Brazil). With the patient seated and utilizing a nasal clip, every respiratory pressure was measured three times with resting interval of 1 min between them, the higher grade value achieved being considered. The difference between the three maneuvers should be lower than 10%. MEP was measured from the total pulmonary capacity (TPC), and both the buccal aerial flow and use of buccinator muscles were avoided. MIP was evaluated from the residual volume (RV), and, then, a maximal inspiration up to TPC was developed. The calculation of the pressures was developed according to the values professed by Neder (2002),Citation18 in agreement with the Guidelines of Brazilian Society of Pneumology and Phthisiology.Citation19
For the analysis of pulmonary function, the forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and Tiffeneau index (FEV1/FVC) were evaluated with a portable spirometer (Micro Plus, UK). With the patient comfortably seated, using a nasal clip, and maintaining erect his trunk, it was requested his/her faster, stronger, and more complete possible expiration, until the total expulsion of pulmonary air, in the RV level. Then, a maximal inspiration up to TPC was developed, with the mouthpiece placed between the teeth and connected to spirometer. Three maneuvers were developed in every measurement and the higher value was considered, which should be lower than 10%.Citation19
Tolerance to submaximal exercise was measured with step test (ST) for 4 min of time, quantifying the number of steps achieved (NSA) in four times (ascend, ascend, descend, descend). Before the test development, the participant developed a pre-test, which consisted in individual familiarization, being the rhythm of ascents and descents equal to those afterward developed.Citation19 The bench was built in resistant wood and covered with nonskid material, in the following dimensions: 15 cm of height, for 40 cm of deep and 60 cm of width.Citation20,Citation21 For the test development, the patient utilized a frequency meter (fitness polar, Finland) positioned above the xiphoid process and below the nipples, controlling the cardiac frequency in a digital watch coupled to the arm opposite to arteriovenous fistula.
The results of urea, hemoglobin, potassium, calcium, and phosphorus concentration, as well as the hematocrit, were verified in the levels of blood exams developed monthly, in the HD unit.
With respect to the quality of life measurement, the patients answered the Kidney Disease and Quality-of-Life—Short-Form version 1.3 (KDQoL-SF 1.3), which includes some questions of the generic questionnaire SF-36 (Medical Outcomes Short Form Study 36) and a specific part about renal disease, composed of items divided into 11 dimensions. The scores in every dimension vary between 0 and 100, with the higher scores reflecting a better quality of life.Citation22,Citation23
Exercise Programs
The participating patients were divided into three groups: group 1—control (G1); group 2—strength (G2), and group 3—aerobic (G3). The group’s selection was randomized into three blocks, in aleatory form through 45 envelops, without external marks, which were jumbled and numbered from 1 to 15, containing inside a sheet with the pertaining group name.Citation22 The choice of patients’ shift obeyed the logistical issues of research execution.
The exercise programs of G2 and G3 were developed during 8 weeks, with the patient seated in the dialysis seat during the first 2 h of HD, at the frequency of three times a week. G1 were evaluated before and after 8 weeks and did not receive any type of intervention or training, but only the evaluations.
G2 were subjected to a peripheral musculature training composed by three series of 15 repetitions in every lower limb. The program was composed of two active exercises of knee flexion-extension, and hip and knee flexion with dorsiflexion of foot, both resisted by anklet, utilizing 40% of load of a one repetition maximum (1RM).Citation23 The 1RM test was developed in the seated position with feet resting on the floor, at an angle of 90˚, straight and recumbent column, arms resting in the dialysis seat support, where the patient executed a knee flexion-extension in complete amplitude, starting the repetition without load for adaptation; further, this movement was started with 0.5 kg, with gradual weight increase in steps of 0.5 kg, with resting interval of 1 min between the series, until the patient achieved the weight in which he/she did not succeed in developing the maximal amplitude, or felt important pain and/or tiredness. The 1RM load was evaluated every 15 days and adjusted to exercises in an individualized way, according to subjective effort perception on the modified scale of Borg.Citation7,Citation24
G3 pedaled on an ergometric bicycle (Biocycle 2700 movement, Brazil) for 20 min, with progressive individualized load intensity, according to subjective effort perception measured on the modified Borg scale, which should remain between the values of 2 and 3 (mild to moderate).Citation17,Citation25,Citation26
G2 and G3 exercises were monitored “before,” “after ten minutes,” and at “exercise protocol termination,” and the following parameters were also verified: “systolic” and “diastolic” blood pressures by means of an aneroid sphygmomanometer (Glicomed, Brazil) and stethoscope (Littmann); heart rate, which should be in the level of 70% of the maximal heart rate (MHR); and, finally, the peripheral oxygen saturation (SpO2) through a finger pulse oximeter (Nonin® Onyx®, Model 9500 finger pulse oximeter, USA).Citation27 The patients were instructed to interrupt the exercises in the presence of both “hypertension” or “hypotension” above the initial status (<200/110 mmHg or >90/70 mmHg), as well as an “SpO2 falling” below 89%, and also in presence of the following signals and symptoms: “headache,” “nape pain,” “chest pain,” “nausea,” “vertigo,” “intense muscular fatigue,” “cramps,” or any other debilitating muscular symptom.Citation28 At exercise termination, peripheral muscles’ (“ischiotibial” and “triceps surae”) passive elongations were developed, maintained for 20 s with two series for every limb, and finalized with passive slipping in the triceps sural muscle.Citation7,Citation17
Statistical Analysis
Data were analyzed with the SPSS 15.0 (Statistical Package for Social Sciences) software, version 13.0, and the quantitative variables were expressed in average and standard deviation. One-way analysis of variance (ANOVA) was applied for comparison between groups with Tukey’s post-hoc test, when the variables presented parametric distribution. To evaluate the intragroup comparison between quantitative variables, Student’s t-test was developed for the matched samples, according to the normality of every variable. The adopted significance level was of 5% (p < 0.05).
RESULTS
The sample characteristics are demonstrated in , with homogeneity being observed between all studied groups, except for the parameter BMI, in the aerobic group.
Table 1. Sample characteristics.
We observed a statistically significant decrease in the MIP (p < 0.05) and MEP (p < 0.05) values compared to what was anticipated, indicating respiratory muscular weakness. The FEV1 and FVC were lower than predicted in the three evaluated groups (), but within the normality range. We did not observe significant alterations when we evaluated the blood pressure of patients at the beginning and end of the study (p > 0.05).
Table 2. Intragroup analysis: respiratory muscles strength, pulmonary function, and tolerance to submaximal exercise.
Table 3. Blood biochemistry.
When all groups were compared after training, we verified () a statistically significant increase in the MIP, MEP, and NSA values, but only in the groups that developed training (G2 and G3) as compared to G1.
In the pre- and post-exercises comparison (), there was a statistically significant increase of MIP and NSA in the trained patients (G2 and G3 groups), as compared to nontrained ones (G1 group). No statistical difference was observed in the spirometry values in all the studied groups.
In the blood examinations of samples collected before and after the physical training program, a statistically significant decrease of urea was observed in the group that developed aerobic training ().
In the quality of life questionnaire specific for renal disease (KDQoL-SF 1.3), we observed in the trained groups (G2 and G3) a statistically significant improvement in the comparison before and after 8 weeks of training, as compared to the control group (G1). In this comparison, the strength group (G2) improved the quality of life in the following domains: social support (p < 0.001), patient satisfaction (p < 0.01), and general health (p < 0.007); the aerobic group (G3) improved in the domains referring to physical functioning (p < 0.05), pain (p <0.05), symptoms (p < 0.04), sleeping (p < 0.006), sexual function (p < 0.01), and energy/fatigue (p < 0.02).
DISCUSSION
The impact of CRF and its treatment could lead to multiple and complex organic dysfunctions, causing the dissemination of several symptoms, such as cardiorespiratory and musculoskeletal ones, and these symptoms could stir up cardiovascular morbidities.9 In this study, we observed that 81.2% of the 32 patients presented arterial hypertension, this result being similar to comorbidities reported by other investigators.Citation29,Citation30
The cardiovascular dysfunctions of patients under HD could decrease the tolerance to exercises in 50% of the anticipated value, supposedly due to musculoskeletal catabolism and intramuscular fat accumulation; these events interfere with both types of stimulation: the sympathetic one, thus inhibiting the chronotropic effect, and the respiratory one, thus decreasing the respiratory muscular strength and the “pulmonary function.”Citation30–Citation32
In this study, the patients presented both MIP and MEP 56% and 59% lower, respectively, than previously anticipated in the earlier evaluation of the three study groups. Similar results were presented in another study, with reduction of 52.9% in the MIP, and of 42.8% in the MEP.Citation33 However, the cause of this weakness and the grade of functional jeopardy are not clear in the literature,Citation34 but a relationship with uremic myopathy, carnitine deficiency, vitamin D, and excess of parathormone is suggested.Citation9,Citation16,Citation35,Citation36
The hypotrophy of type II fibers, myofibrillar ATPase, jeopardy of energetic metabolism due to lower capacity to stimulate the pyruvate dehydrogenase activity, decrease in the utilization of fat as metabolic source, chronic inflammation with cytokine alterations, and intramuscular fat produced by dialysis are also mentioned as factors initiating muscular weakness.7,16,29,36,37
A study emphasizes that children and teenagers with CRF already present respiratory muscular weakness with conservative treatment only, based on the hypothesis that air flow limitation results from muscular strength decrease, which could retard the contractile function of muscular fibers.Citation38
It is yet emphasized that the higher the dialytic period of time, the lower are the spirometric values observed in CRF.Citation39 The average HD time in our study, in the three groups, corresponded to 6.5 ± 4.6 years, and 40% of the 32 patients presented spirometric values lower than the anticipated ones, but within the normality range. There were no statistically significant differences in the spirometry values at pre- and post-training analyses. Similar results, in a study with 33 patients, were also observed; a mild restrictive standard occurred in 21% of the patients, followed by obstructive jeopardy in 6% of the patients after HD, and mixed ventilatory disturbance in the remaining patients.Citation32
These alterations in the pulmonary function could be attributed as much to muscular weakness as to aerial obstruction and arrest, both caused by overload of liquid in the interstitial space and airways, with repeated episodes of pulmonary irritation and bronchoconstriction due to dialysis membrane bio-incompatibility, which could damnify the capillary-alveolar wall, affect the diffusion, induce interstitial fibrosis, and decrease the functional capacity.Citation8,Citation28,Citation31
To evaluate the functionality of patients under HD, and thanks to space scarcity in the site, we have applied in this study the ST following the rhythm determined by the patient himself/herself (self-paced step test), verifying the total number of steps ascended in 6 min, as a marker of “work.” Despite the extensive experience with ST in patients with chronic obstructive pulmonary disease (COPD) and heart failure (HF), in bearers of chronic renal disease (CRD) it is still little utilized, despite to be indicated.Citation19,Citation21
The functional performance and respiratory muscular strength, evaluated respectively by NSA (step test) and manovacuometry (MIP), exhibited a statistically significant increase in the trained groups (G2 and G3) in the pre- and post-interventional analyses. Even though we have not developed, in this study, a specific training of respiratory musculature in G2 and G3, an improvement of inspiratory muscular strength in both groups occurred, possibly due to the work imposed on musculature during the exercise protocols for peripheral muscles. It is emphasized that in CRF, a direct correlation between the functional capacity and the respiratory muscles’ strength effectively occurs,31 which could thus justify our results.
There is evidence that the practice of physical exercises developed during the first 2 h of HD could improve the functionality and muscular strength of patients with CRD, minimize the sympathetic nervous system reflex hypotension, and increase the tolerance to exercise.1,4 These results were also observed in the trained groups of this study (G2 and G3), which exercised during the first 2 h of dialysis.
The majority of studies indicate that exercise protocols with resistance for peripheral muscles could, effectively, increase the volume of muscular fibers resistant to fatigue, the captation and transportation of oxygen by muscles, their capacity to oxidize and metabolize glucose, as well as the respiratory and peripheral muscles’ strength.Citation17,Citation31,Citation33,Citation37,Citation39 Such events could thus justify the results of this study for the strength group (G2), which showed increase in the inspiratory and expiratory strength, as well as the functional capacity at ST in the pre- and post-training comparison.
An exercise protocol similar to that of this study was developed, with strength training, in 49 patients during HD, for 12 weeks; that protocol focused on upper and lower limbs and verified significant increase in muscular strength, measured with a dynamometer, in the quadriceps musculature.7
On the other hand, many recommendations of exercise during HD are referred to 30 min of aerobic activity, with intensity between 70% and 85% of maximal heart rate; however, it is known that the majority of dialyzed patients present decrease in sympathetic stimulation.Citation40,Citation41 Because of that, in our study, the 20 min of aerobic activity were monitored according to individual effort perception (modified Borg scale). Other studies using similar protocols have also verified that such patients can exhibit good response to training, being monitored by the individualized sensation of effort.Citation33,Citation42–Citation44 These studies reinforce our findings, and emphasize the importance of inclusion and continuity of exercise programs for HD patients, aiming at a decrease in sedentary behavior.Citation45
Other studies emphasize that a good physical exercise program, developed during HD, could decrease uremia and improve this population quality of life.Citation46,Citation47 We have also observed a significant decrease of urea (p = 0.032) in G2, at pre- and post-exercise comparisons. Similar results were verified with aerobic training on ergometric bicycle, for 15 min, during the first 3 h of HD, three times a week for 8 weeks.Citation12 One of the hypotheses attributes these results to the above 15 min’ duration of exercise, which could increase the systemic and muscular blood flow, favoring the balance between prostaglandins and thromboxane hormones, which stimulates the peripheral vasodilation and promotes toxin clearance.Citation48–Citation50 Supposedly, such metabolic alterations influence the adenosine triphosphate (ATP), phosphocreatine (PCr), and glycogen concentrations, being the substrate generators of energy for muscular contraction, and able to affect the oxidative capacity.Citation17,Citation29
All of these alterations affect the HD patients’ perception with respect to their quality of life (QoL), which could also improve with the practice of physical exercises,9 decreasing the tiredness and fatigue signals and symptoms, and the cramps manifested by dialysis.1,4 So, we observe in this study a significant improvement in QoL, mainly in the aerobic group, on intragroup analysis. Possibly, these results are attributable to the improvement in lactate production, caused by aerobic exercise that inhibits the glycolytic enzymes, increases the muscular sensitivity to insulin, and decreases the carbohydrate metabolism, which is responsible for the pain complaints, weakness, and peripheral muscles’ fatigue,4,20 and could also justify the significant decrease of pain referred by G2 (p < 0.05) in the intergroup analysis.
Study Limitations
This study has some limitations such as (1) sample size that can limit the results; (2) the average age of the patients, which may be lower than other populations in HD, limiting the extrapolation of results; and (3) the lack of a group practicing both strength and aerobic exercises to compare the existing variables in the groups. Besides, other studies are necessary to determine whether these interventions improve the survival of these patients, and the time period for which the exercise results remain active. For that, it is necessary to create intervention strategies to promote the inclusion of exercise and rehabilitation programs in the treatment protocols, in their dialytic routines, in order to subside the building of health policies addressed to chronic renal patients under HD, in Brazil.
In conclusion, the strength and aerobic exercises developed during HD can improve the respiratory muscular strength, functional performance (step test), and quality of life, when compared to individuals presenting the disease who have not developed any type of physical training. Aerobic exercises, besides these benefits, can also increase the urea clearance.
ACKNOWLEDGMENTS
The authors are grateful to the medical team, patients, and staff of Nefroclínica of Foz do Iguaçu, state of Paraná, especially to nephrologist physicians Marcelo Augusto Gonçalves, Jaime Valdemar Borger, Marta Vaz Dias de Souza Boger, Célia Regina Garcia Barufatti, and Marcelo Eduardo Alfieri, for their confidence, for the opportunity, and because they enabled the development of this investigation.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- National Kidney Foundation. KDOQI clinical practice guidelines for chronic kidney disease. Am J Kidney Dis. 2002;39(Suppl. 1):1–246.
- Marques AB, Pereira DC, Ribeiro R. Motivos e frequência de internação dos pacientes com insuficiência renal crônica em tratamento hemodialítico. Arq Ciênc Saúde. 2005;12:67–72.
- Dummer CD, Thome FS, Veronese FV Doença renal crônica, inflamação e aterosclerose: novos conceitos de um velho problema. Rev Assoc Med Bras. 2007;53:446–450.
- Mustata S, Chan C, Lai A, Miller J. Impact of an exercise program on arterial stiffness and insulin resistance in hemodialysis patients. J Am Soc Nephrol. 2004;15:2718.
- Reboredo MM, Henrique DMN, Bastos MG, Paula RB. Exercício físico em pacientes dialisados. Rev Bras Med Esporte. 2007;13:427–430.
- Medeiros RH, Meyer F. Impacto da insuficiência renal crônica no perfil físico do individuo em hemodiálise. Revista Perfil. 2001;5:41–48.
- Cheema B, Abas H, Smith BProgressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007;18:1594–1601.
- Blake C, O’Meara MY. Subjective and objective physical limitations in high-functioning renal dialysis patients. Nephrol Dial Transplant. 2004;19(19):3124–3129.
- Coelho CC, Aquino ES, Lara KL, Peres TM, Barja PR, Lima EM. Repercussões da insuficiência renal crônica na capacidade de exercício, estado nutricional, função pulmonar e musculatura respiratória de crianças e adolescentes. Rev Bras de Fisiot. 2008;12:1–6.
- Pierson D. Respiratory considerations in the patient with renal failure. Respir Care. 2006;51:413–422.
- Johansen KL, Finkelstein FO, Dennis A, Revicki MG, Christopher E, Tracy J. Systematic review and meta-analysis of exercise tolerance and physical functioning in dialysis patients treated with erythropoiesis-stimulating agents. Am J Kidney Dis. 2010;55:535–548.
- Parsons TL, Toffelmire EB, King-Vanvlack CE. Exercise training during hemodialysis improves dialysis efficacy and physical performance. Arch Phys Med Rehabil. 2006;87:680–687.
- Cheema BSB, O’Sullivan A, Chan MProgressive resistance training during hemodialysis: rationale and method of a randomized-controlled trial. Hemodial Int. 2006;10:303–310.
- Depaul V, Morelande J, Eager T, Clase CM. The effectiveness of aerobic and muscle strength training in patients receiving hemodialysis and EPO: a randomized controlled trial. Am J Kidney Dis. 2002;40:1219–1229.
- Kouidi E, Albani M, Natsis KThe effects of exercise training on muscle atrophy in hemodialysis patients. Nephrol Dial Transplant. 1998;13:685–699.
- Storer TW, Casaburi R, Sawelson S, Kopple JD. Endurance exercise training during hemodialysis improves strength, power, fatigability and physical performance in maintenance hemodialysis patients. Nephrol Dial Transplant. 2005;20:1429–1437.
- Painter P, Carlson L, Carey S, Paul SM, Myll J. Physical functioning and health-related quality-of-life changes with exercise training in hemodialysis patients. Am J Kidney Dis. 2000;35:482–492.
- Neder JA, Andreoni S, Lerario MC, Nery LE. Reference values for lung function tests. II. Maximal respiratory pressures and voluntary ventilation. Braz J Med Biol Res. 1999;32:719–727.
- SBN. Sociedade Brasileira de Pneumologia e Tisiologia. Diretrizes para testes de função pulmonar. J Pneumol. 2002;28:1–238.
- Karsten M. Proposta de um teste de exercício submáximo, com a utilização de banco e cadência livre Dissertação Mestrado em Centro de Educação Física, Fisioterapia e Desportos, Universidade Estadual de Santa Catarina, Florianópolis, 2003; 114.
- Brunelli A. Stair climbing test predicts cardiopulmonary complications after lung resection. Chest. 2002;121(4):1106–1110.
- Hays RD, Kallich JD, Mapes DL, Coons SJ, Amin N, Carter WB. Kidney Disease Quality of Life Short Form (KDQOL-SF TM). Version 1.3: a manual for use and scoring. Santa Monica. 1997;1:1–39.
- Materko W, Neves C, Santos EL. Modelo de predição de uma repetição máxima (1RM) baseado nas características antropométricas de homens e mulheres. Rev Bras Med Esporte. 2007;13:27–32.
- Borg GAAdministração das escalas de Borg. In: Borg GA, ed. Escalas De Borg Para a Dor E O Esforço Percebido. São. Paulo: Manole; 2000.
- Williams F, Frances SM, Leitner M. Exercise training and heart failure: a systematic review of current evidence. J Br Gen Pract. 2002;52:47–55.
- Kirsten PK, Robert GF, James ES, Jeff SC, Andrew DW. Intradialytic versus home based exercise training in hemodialysis patients: a randomised controlled trial. BMC Nephrol. 2009;10:1–6.
- Bush A, Gabriel R. Pulmonary function in chronic renal failure: effects of dialysis and transplantation. Thorax. 1991;46:424–428.
- Kalender B, Erk M, Pekpak MThe effect of renal transplantation on pulmonary function. Nephron. 2002;90:72–77.
- Johansen KL, Painter P. Exercise for patients with CKD: what more is needed? (Guest editorial). Adv Chronic Kidney Dis. 2009;16:407–409.
- Cury JL, Brunetto AF, Aydos RD. Efeitos negativos da insufciência renal crônica sobre a função pulmonar e a capacidade funcional. Rev Bras Fisioter. 2010;14:91–98.
- Dipp T, Silva AMV, Sgnori LUForça Muscular Respiratória e Capacidade Funcional na Insuficiência Renal Terminal. Rev Bras Med Esporte. 2010;16:246–249.
- Siafakas N, Argyrakopoulos T, Andreopoulos K, Tsoukalas G, Tzanakis N, Bouros D. Respiratory muscle strength during continuous ambulatory peritoneal dialysis (CAPD). Eur Respir. 1995;8:109–113.
- Rodrigues MG, Castro AM, Coelho DC. Respiratory function evaluation and rehabilitation of patients with chronic renal insufficiency under hemodialysis. Eur Resp J. 2000;16:505.
- Johansen KL, Kaysen GA, Hung AM, Silva M, Chertow GM. Longitudinal study of nutritional status, body composition, and physical function in hemodialysis patients. Am J Clin Nutr. 2009;77:842.
- Saiki JK, Varizi ND, Naeim F, Meshkinpour H. Dialysis-induced changes in muscle strength. J Dial. 1980;4:191–201.
- Clanton TL, Dixon GF, Drake J, Gadek JE. Effects of breathing pattern on inspiratory muscle endurance in humans. J Appl Physiol. 1985;59:1834–1841.
- Weiner P, Zidan F, Zonder HB. Hemodialysis treatment may improve inspiratory muscles strength and endurance. J Med Sci. 1997;33:134–138.
- Coelho MC, Castro AM, Tavares HÁEfeitos de um programa de exercícios físicos no condicionamento de pacientes em hemodiálise. J Bras Nefrol. 2006;3(28):121–127.
- Bardin T. Musculoskeletal manifestations of chronic renal failure. Curr Opin Rheumatol. 2003;15:48–54.
- Kosmadakis GC, Bevington A, Smith ACPhysical exercise in patients with severe kidney disease. Nephron Clin Pract. 2010;115:7–16.
- Fukuta H, Hayano J, Ishihara SPrognostic value of heart rate variability in patients with end-stage renal disease on chronic hemodialysis. Nephrol Dial Transplant. 2003;18:318–325.
- Konstantinidou E, Koukouvou G, Kouidi E, Deligiannis A, Tourkantonis A. Exercise training in patients with end-stage renal disease on hemodialysis: comparison of three rehabilitation programs. J Rehabil Med. 2002;34:40–45.
- Parfrey PS, Wish T. Quality of life in CKD patients treated with erythropoiesis-stimulating agents. Am J Kidney Dis. 2010;55:423–425.
- Blake C, O’Meara YM. Subjective and objective physical limitations in high-functioning renal dialysis patients. Nephrol Dial Transplant. 2004;19:3124–3129.
- Sakkas GK, Sargeant AJ, Mercer THChanges in muscle morphology in dialysis patients after 6 months of aerobic exercise training. Nephrol Dial Transplant. 2003;18:1854–1861.
- Headley S, Germain M, Mailloux PResistance training improves strength and functional measures in patients with end stage renal disease. Am J Kidney Dis. 2002;40:355–364.
- Violan MA, Pomes T, Maldonado SExercise capacity in hemodialysis and renal transplant patients. Transplant Proc. 2002;34:417–418.
- Karamouzis L, Grekas D, Karamouzis MPhysical training in patients on hemodialysis has a beneficial effect on the levels of eicosanoid hormone-like substances. Hormones. 2009;8:129–137.
- Parsons TL, Toffelmire EB, Vanvlack K. The effect of an exercise program during hemodialysis on dialysis efficacy, blood pressure and quality of life in end-stage renal disease (ESRD) patients. Clin Nephrol. 2004;61:261–274.
- Johansen KL, Painter P, Sakkas GKEffects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: A randomized, controlled trial. J Am Soc Nephrol. 2006;17:2307–2314.