Abstract
Tubulointerstitial fibrosis is a common pathway that leads to kidney failure, and persistent tubulointerstitial inflammation is a key event in the development of tubulointerstitial fibrosis. The new immunosuppressive drug FTY720 modifies lymphocyte migration into injured tissues by sequestering lymphocytes within secondary lymphoid organs. However, its therapeutic effect on tubulointerstitial inflammation and fibrosis had not been well understood. This study was designed to explore the effect of FTY720 on tubulointerstitial inflammation and fibrosis in subtotally nephrectomized (SNX) rats. In total, 24 male Sprague–Dawley rats were used. Seven days after 5/6 nephrectomy, rats were randomized to FTY720 (1 mg/kg/d) and placebo-treated groups. Sham-operated rats served as controls. FTY720 significantly attenuated the rise in proteinuria, serum creatinine, urea nitrogen and N-acetyl-β-D-glucosaminidase activity in SNX rats, and reduced the count of peripheral white blood cells and lymphocytes in SNX rats. Morphological analysis revealed that there was severe tubulointerstitial inflammation and fibrosis in SNX group and much more tubulointerstitial infiltrating inflammatory cells with high expression of CD3, CD4, CD8, CD20, CD68, CD163 and CCR-7 in SNX group, as compared with the controls, but the lesions were attenuated significantly by treatment with FTY720. Furthermore, the expressions of proinflammatory molecules (IL-6, TNF-α and MCP-1), profibrotic molecule (TGF-β1) and production of extracellular matrix proteins such as fibronectin and types I and III collagens were upregulated in SNX rats. FTY720 administration significantly reduced these abnormalities. In summary, FTY720 exerts therapeutic effects on tubulointerstitial fibrosis in SNX rats by inhibiting the tubulointerstitial inflammatory response.
Introduction
Renal fibrosis is the principal process underlying the progression of chronic kidney disease (CKD) to end-stage renal disease (ESRD).Citation1 It involves glomerulosclerosis, tubulointerstitial fibrosis and vascular sclerosis. Tubulointerstitial fibrosis (TIF) has been regarded as the most consistent predictor of an irreversible loss of renal function and progression to ESRD.Citation2 Renal interstitial inflammation is closely related to the progressive renal fibrosis. Inflammatory infiltrates in chronic renal disease are composed of heterogeneous cell types, such as macrophages and lymphocytes.Citation3 Although some cells are derived from the proliferation of resident macrophages, the majority enter the kidney from the circulation. Macrophage infiltration is driven by the local expression of molecules, such as monocyte chemoattractant protein-1 (MCP-1).Citation4 Infiltrating cells release proinflammatory cytokines, such as TNF-α, IL-6 and IL-1β, and profibrotic molecules such as TGF-β1.Citation5 These factors in turn activate interstitial resident fibroblasts to produce extracellular matrix proteins, such as fibronectin and types I and III collagens.Citation6
Halting the process of renal inflammation and fibrosis remains a huge challenge for nephrologists. FTY720 {fingolimod; 2-amino-2-[2-(4-octylphenyl)ethyl]-1,3- propanediol hydrochloride} is a sphingosine-1-phosphate (S1P) analog originally developed as a novel immunosuppressant and has currently been tested in phase III studies for the treatment of renal transplantation.Citation7 The mechanism of immunosuppressive action of FTY720 has not been fully elucidated, although a characteristic depletion of circulating lymphocytes in the peripheral blood and a reduction of lymphocyte infiltration into injured tissues by accelerating lymphocyte homing into peripheral lymph nodes has been observed in some experimental models.Citation8 More interestingly, previous studies have shown that FTY720 attenuates renal injury in many experimental models, such as anti-thy1-induced chronic glomerulosclerosis,Citation9 renal ischemia reperfusion injury,Citation10 subtotal nephrectomy,Citation11 experimental hypertensive nephropathyCitation12 and antiglomerular basement membrane glomerulonephritis.Citation13 Tubulointerstitial inflammation and fibrosis are common pathways of CKD leading to ESRD, regardless of the underlying cause. There are insufficient reports on the role of FTY720 in tubulointerstitial inflammation and fibrosis. Therefore, a more detailed insight into the potential pathomechanism is of interest to investigators. This study was designed to explore the effect of FTY720 on tubulointerstitial inflammation and fibrosis in 5/6 subtotal nephrectomized rats.
Materials and methods
Animal experiments
In total, 24 male Sprague–Dawley rats (Shanghai Super-B&K Laboratory Animal Corp. Ltd, Shanghai, China) with an initial weight of 200–220 g were housed in pairs in cages at a constant room temperature (22 °C) and humidity (55%) under a 12:12 h light–dark cycle. Chronic renal failure was initiated by a one-step subtotally nephrectomy as described previously.Citation14 In brief, the right kidney was removed and a weight-controlled 2/3 left-side nephrectomy was performed (according to the weight of the left kidney). Sham operation was done by laparotomy and bilateral decapsulation of the kidneys. The rats had free access to tap water. The experiments were performed according to the Chinese law on animal protection.
After 7 d of surgery, the animals were divided into the following groups: sham + vehicle (Sham, n = 8), 5/6 subtotal nephrectomy + vehicle (SNX, n = 8) and SNX + FTY720 (FTY720, n = 8, FTY720 was purchased from Selleck Chemicals (Houston, TX). Treatment with FTY720 was started at the first week after complete surgery and the drug was administered by gavage in a dose of 1 mg/kg/d in sterile water.Citation9,Citation11,Citation15 Systolic blood pressure was measured on Weeks 0, 2, 4, 6, 8, 10 and 12 after the subtotal nephrectomy in conscious animals by tail-cuff plethysmography and calculated as an average of three separate measurements at each session. Rats were killed on the 12th week after surgery. Before killed, rats were housed in metabolic cages for 24-h urine collection. Blood samples were collected by cardiac punctureand examined for white blood cell counts, blood urea nitrogen (BUN) and serum creatinine (Scr). Urine samples were examined for 24-h proteinuria and N-acetyl-β-D-glucosaminidase activity (NAG). White blood cell counts, BUN and Scr were measured at the Clinical Biochemistry Department of Zhong Da Hospital by automatic analyzers (Hitachi, Tokyo, Japan). NAG activity was determined from urine sample using an assay kit (Jiancheng, Nanjing, China).Citation16
Tissue preparation
Rats were perfused with a saline solution containing 0.5 g/L of procaine hydrochloride at a pressure of 120–140 mm Hg by the retrograde insertion of a cannula into the abdominal aorta before sacrifice. After perfusion, the renal tissue was weighed, and several midcoronal sections were fixed in 10% buffered formalin and embedded in paraffin for histological investigation and immunohistochemical analysis. Pieces of kidneys were snap-frozen and stored at −80 °C for protein extraction.
Morphological analysis of the kidney
For the quantitative evaluation of a number of interstitial inflammatory cells, periodic acid-Schiff (PAS) staining sections were analyzed using a grid containing 100 fields. The interstitial cells per grid were counted in 20 nonoverlapping tubulointerstitial areas.Citation11 For the evaluation of the percentage of tubulointerstitial fibrosis, Masson-stained sections were analyzed, and the severity of tubulointerstitial fibrosis was estimated.Citation17
Immunohistochemistry
The following antibodies were used for immunohistochemical staining: polyclonal goat anti-rat CD3 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA), polyclonal rabbit anti-rat CD4 (Santa Cruz Biotechnology Inc.), polyclonal rabbit anti-rat CD8 (Santa Cruz Biotechnology Inc.), polyclonal goat anti-rat CD20 antibody (Santa Cruz Biotechnology Inc.), monoclonal mouse anti-rat ED-1 (CD68) antibody (Santa Cruz Biotechnology Inc.), CCR7 (Epitomics, CA), monoclonal mouse anti-rat ED-2 (CD163) (Santa Cruz Biotechnology Inc.), polyclonal goat anti-rat IL-6 (Santa Cruz Biotechnology Inc.), polyclonal goat anti-rat TNF-α (Santa Cruz Biotechnology Inc.), polyclonal rabbit anti-rat MCP-1 (Santa Cruz Biotechnology Inc.), polyclonal rabbit anti-rat TGF-β1 (Santa Cruz Biotechnology Inc.), polyclonal rabbit anti-rat collagen I (Santa Cruz Biotechnology Inc.), polyclonal rabbit anti-rat collagen III (Santa Cruz Biotechnology Inc.) and polyclonal rabbit anti-rat fibronectin (Santa Cruz Biotechnology Inc.).
The sections were deparaffinized in xylene and rehydrated in graded ethanol. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxidase in methanol for 30 min at room temperature. The sections were microwaved for 5 min to retrieve the antigen by the antigen unmasking solution. After incubation with 5% skimmed milk for 1 h at room temperature, the sections were incubated with the primary antibodies overnight at 4 °C. Then, the tissues were washed several times in phosphate buffer saline (PBS) and incubated for 45 min with the secondary antibody; subsequently, the tissues were visualized with a Diaminobenzidine (DAB) kit, examined under a microscope and photographed.
For the evaluation of the numbers of the T lymphocytes (CD3), T helper cells (CD4), cytotoxic T cells (CD8), B lymphocytes (CD20), macrophages (ED-1/CD68) and macrophage subtypes (CCR7/M1, CD163/M2), interstitial cross-sections were analyzed using a grid containing 100 fields (Leica). In each kidney, at least 30 nonoverlapping cortical areas from two different sections were evaluated.Citation11 For the immunoperoxidase stains to detect TGF-β1, 30 interstitial areas were graded by a qualitative score of 0–3 according to the intensity of the immunostaining (0 = no staining, 1 = low intensity, 2 = medium intensity and 3 = high intensity). Expression for collagens I, III and fibronectin in tubulointerstitium was analyzed by randomly selecting at least 15 cortical sections/animal using ×200 magnification. Matrix protein expression is indicated by the percentage of the positive-staining area per tubulointerstitium.Citation18
Western blot analysis
Proteins from the kidney cortex extracts were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred onto polyvinylidene difluoride membranes (Millipore, MA). Nonspecific antibody binding was blocked by a pre-incubation of the membrane in 1× Tris-buffered saline containing 5% skim milk for 1 h at room temperature. The membranes were then incubated overnight at 4 °C with the primary antibodies against IL-6, TNF-α and MCP-1, followed by incubation with the appropriate peroxidase-conjugated secondary antibodies for 1 h at room temperature. Finally, the signals were detected using an enhanced chemiluminescence advanced system (GE Healthcare, Chalfont St. Giles, UK).
Statistical analysis
The results are presented as the mean ± SD. Comparisons among several groups were determined with one-way analysis of variance (ANOVA) using SPSS18.0. If the ANOVA indicated an overall significant difference (p < 0.05), Student–Newman–Keuls (SNK) test was used to determine the significance of a difference in the means between any two groups. A level of p < 0.05 was considered significant.
Results
Effects of FTY720 on physiological and biochemical parameters in subtotal nephrectomized rats
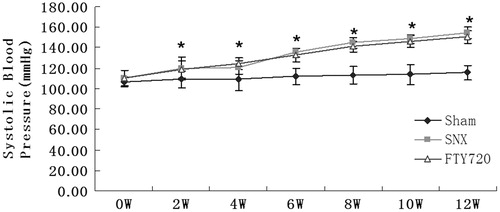
After the subtotal nephrectomy, blood pressure increased significantly in the SNX group compared with the sham group (p < 0.01). FTY720 did not show significant effect on blood pressure in the SNX group compared with the vehicle-treated SNX rats (p > 0.05, ).
Figure 1. Effects of FTY720 on systolic blood pressure in the subtotally nephrectomized rats. After the subtotal nephrectomy, blood pressure rose significantly in the SNX group compared with the sham group. Treatment with FTY720 did not attenuate these abnormalities. n = 8, *p < 0.01 versus sham.
The SNX group exhibited proteinuria, increased plasma urea and creatinine and NAG compared to the sham group (p < 0.01). Treatment with FTY720 significantly attenuated these abnormalities (p < 0.01, ).
Table 1. Effect of FTY720 on basic data in 5/6 nephrectomized rats.
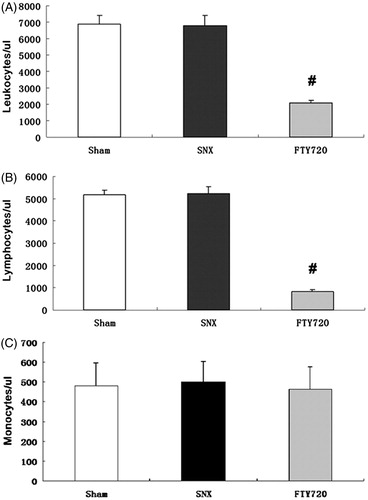
Effects of FTY720 on the peripheral white blood cell count.
In SNX rats, the numbers of circulating leukocytes and lymphocytes were similar to those in the sham animals (p > 0.05). However, the counts of peripheral lymphocytes and leukocytes were significantly reduced by treatment with FTY720 (p < 0.01, ). In SNX rats, the numbers of circulating monocytes were similar to those in the sham animals (p > 0.05), FTY720 did not reduce the number of peripheral monocytes (p > 0.05)
Figure 2. Effects of FTY720 on the number of peripheral white blood cells, lymphocytes and monocytes. The whole white blood cell count (A), blood lymphocyte count (B) and blood monocyte count (C) 12 weeks after the 5/6 nephrectomy. FTY720 significantly reduced the number of peripheral whole white blood cells and lymphocyte, but FTY720 did not reduce the number of peripheral monocytes. #p < 0.01 versus SNX.
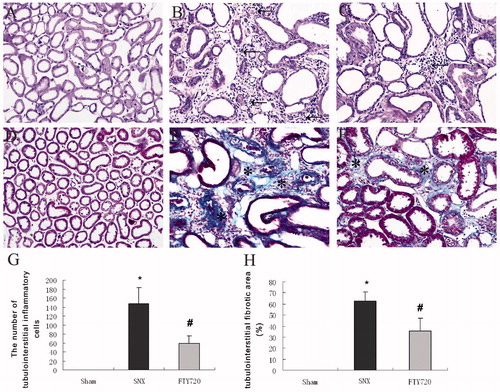
FTY720 attenuates the tubulointerstitial histological changes in subtotal nephrectomized rats
Compared to the controls (), the SNX kidneys showed severe tubular dilatation, tubular atrophy, widened interstitial space and a greater number of inflammatory cells (), which were ameliorated by treatment with FTY720 (). The administration of FTY720 significantly inhibited the infiltration of tubulointerstitial inflammatory cells compared to that in SNX rats (p < 0.01, ). The tubulointerstitial deposition of collagen detected by Masson staining was significantly increased in the SNX rats than that in the sham-operated controls, which was significantly attenuated when treated with FTY720. (p < 0.01, ).
Figure 3. FTY720 attenuates the tubulointerstitial histological changes in the subtotally nephrectomized rats. Representative examples of PAS stains (A–C) and Masson stains (D, E) showing the tubulointerstitial histological changes (original magnification: ×200). Semiquantitative analyses of the tubulointerstitial inflammatory cells (G) and tubulointerstitial fibrosis (H). The tubulointerstitial fibrotic area and the number of inflammatory cells was markedly increased in the SNX animals compared with sham and was lower in SNX+FTY720 group; n = 8, *p < 0.01 versus sham, #p < 0.01 versus SNX.
Effects of FTY720 on tubulointerstitial infiltrating inflammatory cells
In the sham-operated rats, the T lymphocytes (CD3-positive), the subtypes of T helper cells (CD4-positive) and cytotoxic T cells (CD8-positive) were almost undetectable. However, in the SNX group, a markedly increased level of interstitial infiltration by different T cells was detected compared to the sham animals (p < 0.01). The interstitial infiltration by different T lymphocyte subtypes was significantly attenuated by treatment with FTY720 (p < 0.01, ).
Figure 4. Effects of FTY720 on tubulointerstitial staining of T cells (CD3), T cell subtypes (CD4, CD8), B cells (CD20), macrophages (CD68) and macrophage subtypes (CCR7/M1, CD163/M2). FTY720 treatment significantly reduced the number of the interstitial infiltration of CD3-positive (A), CD4-positive (B), CD8-positive T cells (C), CD20-positive B cells (D), CD68-positive (E), CCR7-positive (F) and CD163-positive macrophages (G) in the SNX rats. Original magnification: ×400. Data are presented as the mean ± SD, n = 8, *p < 0.01 versus sham, #p < 0.01 versus SNX.
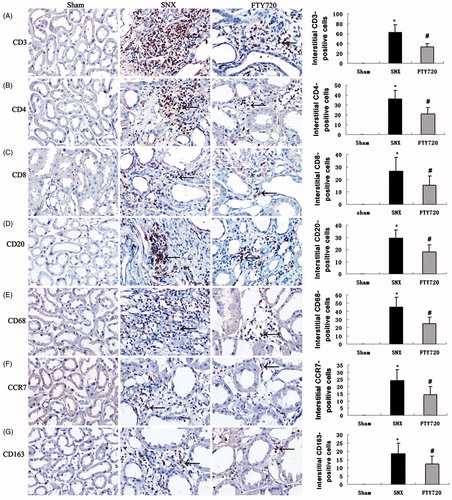
Interstitial B lymphocytes (CD20-positive) were significantly elevated in the SNX group compared with the sham group. Treatment with FTY720 significantly reduced the number of interstitial B lymphocytes in the SNX rats (p < 0.01, ).
The number of interstitial monocytes/macrophages (ED-1/CD68-positive) and their different phenotypes, M1 (CCR7) and M2 (CD163), were significantly increased in the SNX group compared to the sham group (p < 0.01). Treatment with FTY720 markedly reduced the interstitial positive cell counts of monocytes/macrophages and their different phenotypes (M1 and M2) in the SNX rats (p < 0.01, ).
Effects of FTY720 on the expression of proinflammatory and profibrotic molecules
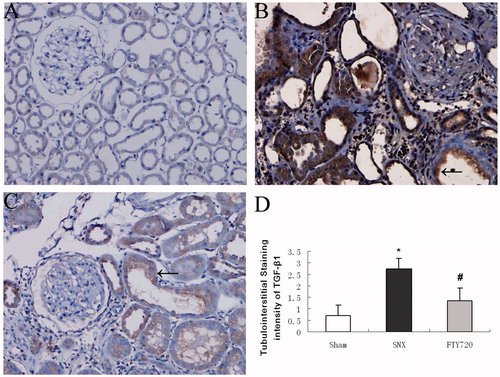
To determine whether FTY720 could reduce the expression of proinflammatory molecules, we analyzed the expression of IL-6, TNF-α and MCP-1 by western blot analysis (). The protein expression of some proinflammatory molecules, such as IL-6, TNF-α and MCP-1 were increased in the SNX group, while FTY720 administration significantly attenuated these abnormalities (p < 0.01, ). The immunostaining for TGF-β1 in the tubulointerstitial space was significantly enhanced in the rats with SNX compared to the sham group, while treatment with FTY720 significantly attenuated the expression (p < 0.01, ).
Figure 5. Effects of FTY720 on the expression of proinflammatory molecules. Representative western blotting analysis of IL-6, TNF-α and MCP-1 are shown for the sham, SNX and SNX+FTY720 groups (A). Densitometric results showed that FTY720 treatment prevented the upregulation of IL-6 (B), TNF-α (C) and MCP-1 (D). β-Actin was used as an internal control. Data are presented as the mean ± SD, n = 3, *p < 0.01 versus sham, #p < 0.01 versus SNX.
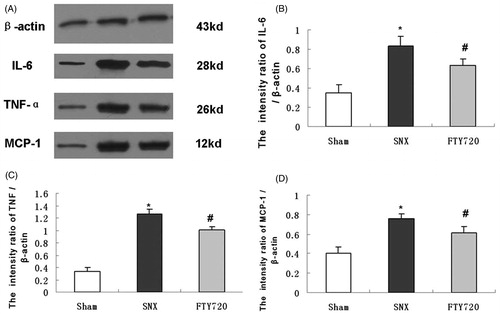
Figure 6. Effects of FTY720 on the expression of profibrotic molecules (TGF-β1). Representative tubulointerstitial immunostaining of TGF-β1 are shown for the sham (A), untreated SNX (B) and SNX+FTY720 groups (C). FTY720 treatment prevented the upregulation of TGF-β1 in the tubulointerstitial area (D). Original magnification: ×200. Data are presented as the mean ± SD, n = 8, *p < 0.01 versus sham, #p < 0.01 versus SNX.
Effects of FTY720 on production of extracellular matrix proteins
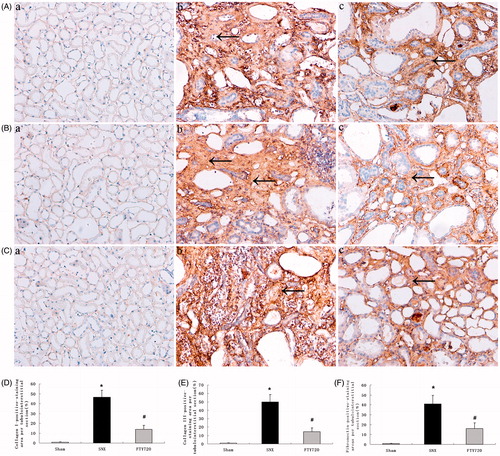
The protein expression of extracellular matrix proteins showed a similar trend as histological fibrosis (). Tubulointerstitial protein deposition of collagens I, III and fibronectin were increased in the SNX group compared to the sham group, while FTY720 administration significantly attenuated these abnormalities (p < 0.01, ).
Figure 7. Effects of FTY720 on protein expression of collagens I, III and fibronectin. Representative tubulointerstitial immunostaining of collagens I (A), III (B) and fibronectin (C) are shown for the sham (a), untreated SNX (b) and FTY720-treated SNX groups (c). Original magnification: ×200. the positive-staining areas of collagens I, III and fibronectin are larger in the SNX rats than in the sham; FTY720 administration significantly reduced these abnormalities (D, E, F). Data are presented as the mean ± SD, n = 8, *p < 0.01 versus sham, #p < 0.01 versus SNX.
Discussion
Tubulointerstitial fibrosis is a common consequence of a diverse range of kidney diseases leading to end-stage renal failure.Citation2 As recently reviewed,Citation3,Citation19 chronic inflammation is one of the key risk factors contributing to tubulointerstitial fibrosis. Therefore, it is hypothesized that inhibiting renal interstitial inflammation is a pivotal strategy to halt the progression of interstitial fibrosis and thus prevent ESRD. In this study, we demonstrated that FTY720 attenuated the infiltration of inflammatory cells into the tubulointerstitial area, inhibited the expression of proinflammatory and profibrotic molecules and blocked production of extracellular matrix proteins in SNX rats. Our results provide novel insight into the use of FTY720 for treating chronic kidney disease by inhibiting tubulointerstitial inflammation and fibrosis.
It is well recognized that inflammation plays a crucial role in the initiation and progression of tubulointerstitial fibrosis after an injury. The classic view on the connection between inflammation and fibrosis is that they are mediated in a paracrine fashion, whereby inflammatory cells secrete proinflammatory and profibrotic cytokines that act on resident fibroblasts and tubular cells to promote fibrogenesis.Citation5 Merely reducing the accumulation of extracellularmatrix (ECM) might not result in an improvement or stabilization of renal function.Citation20 Advanced tubulointerstitial fibrosis represents a vicious cycle of inflammation, tissue damage and fibrosis.Citation21 Furthermore, most therapeutic approaches to renal fibrosis are not specific to fibrosis but rather affect generic processes that act on multiple levels. Therefore, controlling renal inflammation may be the best way to target renal fibrosis and to maintain renal function.
However, the role of inflammation in the progression of fibrosis is more complicated than previously thought. Inflammatory cells exhibit heterogeneous phenotypes and have incredible functional plasticity in the development of kidney diseases.Citation22 It remains largely unclear why and how inflammatory cell subtypes or phenotypes influence the rather unspecific inflammation that accompanies progressive renal diseases. Recently, it was found that lymphocytes, specifically CD4+ T cells, play a critical role in renal fibrosis following a ureteric obstruction.Citation23 In addition, macrophages can switch phenotypes in response to the ever-changing microenvironment and alter their effect from proinflammatory to profibrotic.Citation22 These new data demonstrate the plasticity of inflammatory cell function and call for conceptual and strategic innovations to develop effective cellular-based anti-inflammatory therapies for the treatment of CKD patients. Most immunosuppressive drugs currently used for autoimmune diseases therapy interfere with discrete events during T and B cell activation. The sphingosine-1-phosphate receptor agonist FTY720 could downregulate autoimmune inflammatory responses by modifying the migration of lymphocytes into injured tissues. It is the primary intention of this study to explore the effect of FTY720 on the tubulointerstitial inflammatory response in SNX rats. In this study, we found that FTY720 attenuated the number of lymphocyte cells infiltrating the tubulointerstitial area of SNX animals. The marked reduction of lymphocyte infiltration into the tubulointerstitial area of FTY720-treated rats may be explained by S1P receptor internalization and a downregulation by FTY720.
Although chronic kidney disease is characterized by widespread kidney lymphocyte infiltration, the role of lymphocytes in the progression of tubulointerstitial injury is not clearly defined. It remains largely unclear why and how different kinds of lymphocytes and subtypes of T lymphocytes influence the unspecific inflammation that occurs with progressive renal diseases. Our experiments showed the numbers of the subtypes of T helper cells (CD4) and cytotoxic T cells (CD8) were significantly increased in the SNX group compared to the controls and FTY720 treatment led to a significant reduction. Previously, the relative percentage of B lymphocytes in the renal tubulointerstitium in chronic kidney disease was considered low.Citation24,Citation25 In our study, there was a marked staining for CD-20-positive B cells in the SNX rats compared to the sham-operated controls. FTY720 treatment significantly reduced the infiltration of tubulointerstitial CD-20-positive B cells in the SNX rats.
In this study, we demonstrated that FTY720 treatment could induce a marked lymphopenia, while without any effect on monocyte count. In addition, we also found a reduction of macrophages infiltration in the tubulointerstitium in FTY720-treated SNX rats. Previous studies have indicated that MCP-1 serves as a chemotactic signal to attract macrophages to the injured site in the kidney.Citation26,Citation27 Recruitment of lymphocytes to the kidney could induce the production of MCP-1 by renal cells.Citation28 It is therefore we assumed that the reduction of infiltration of macrophage in the tubular interstitium by FTY720 treatment may relate to the reduced production of MCP-1 in the kidney.
The contribution of macrophages to renal injury is well documented.Citation27 Recent reports indicate that the activation status of infiltrating macrophages is a major determinant of injury.Citation29 When monocytes from circulating blood are recruited to the injured site, they differentiate into two broad but distinct subsets of macrophages that are categorized as either classically activated (M1) or alternatively activated (M2).Citation30 In general, M1 macrophages display a typical proinflammatory phenotype, produce a variety of chemokines and therefore have pathogenic functions that lead to tissue damage. M2 macrophage subpopulations resolve inflammation and repair injury. However, insufficient vascular and epithelial healing, abundant growth factor secretion promotes profibrotic M2 subpopulations that accelerate fibrogenesis.Citation22 It remains largely unclear how macrophage phenotypes determine the progression or resolution of renal inflammation and fibrosis during the different phases of renal injury. CCR7 is a surface marker indicative of an M1 phenotype, and CD163 is a surface marker representative of an M2 phenotype.Citation31 In this study, we found that both M1 and M2 macrophage subsets were markedly increased in the SNX rats compared to the sham group. Treatment with FTY720 significantly inhibited the infiltration of M1 and M2 macrophages, further suggesting that FTY720 might exert its antifibrotic effect by inhibiting the inflammatory process that occurs in injured renal tissue.
A series of proinflammatory and profibrotic molecules secreted by lymphocytes and macrophages directly contribute to renal injury.Citation4,Citation5 There is a complicated interaction between lymphocytes and macrophages in a mutual and complex manner. Previous work has shown a positive correlation between renal injury and the over expression of TNF-α, IL-6 and MCP-1.Citation4 TGF-β1 is a central mediator of fibrosis that works primarily by inducing ECM production and the proliferation of myofibroblasts and fibroblasts.Citation5 Our study showed that FTY720 treatment significantly attenuated the expression of proinflammatory molecules (TNF-α, IL-6 and MCP-1) and profibrotic molecules (TGF-β1) in the SNX group, further suggesting the potential for this treatment to be an anti-inflammatory agent in CKD treatment.
Our results showed that the counts of peripheral lymphocytes and leukocytes were significantly reduced in the SNF group. It naturally causes some concern about its potential side-effects. In an initial phase II study in multiple sclerosis, Kappos et al.Citation32 found that giving fingolimod at dosage of 1.5 or 5 mg daily, no severe infectious complications occurred in the placebo-controlled core phase (6 months). Furthermore, treatment with FTY720 did not promote spontaneous bacterial infections after experimental stroke in mice.Citation33 It is noted that fingolimod-mediated lymphopenia is primarily due to the retention of lymphocytes in lymphatic tissue and is fully reversible in most patients. FTY720 does not seem to repress the function of lymphocytes in the context of the primary immune response.Citation34 However, pharmacovigilance should be practiced when administering FTY720 clinically.
In conclusion, our study demonstrates that FTY720 ameliorates the tubulointerstitial injury in 5/6 nephrectomized rats by inhibiting the infiltration of tubulointerstitial inflammatory cells, the expression of proinflammatory molecules and tubulointerstitial fibrosis. These findings provide novel insights into this newly developed drug with regard to the prevention of the progression of chronic kidney disease.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was financially supported by the National Natural Scientific Fund (no. 81130010), the National Key Basic Research Project (no. 2012CB517700) and the Jiangsu Natural Scientific Fund (no. BK2011061) with Professor Bi-Cheng Liu as the PI.
References
- Eddy AA. Scraping fibrosis: UMODulating renal fibrosis. Nat Med. 2011;17:553–555
- Hewitson TD. Renal tubulointerstitial fibrosis: common but never simple. Am J Physiol Renal Physiol. 2009;296:F1239–F1244
- Liu Y, Chowdhury P, Sacks SH, Sheerin NS, Wong W. Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney Int. 2010;78:351–362
- Eardley KS, Zehnder D, Quinkler M, et al. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int. 2006; 69(7):1189–1197
- López-Hernández FJ, López-Novoa JM. Role of TGF-β in chronic kidney disease: an integration of tubular, glomerular and vascular effects. Cell Tissue Res. 2012;347(1):141–154
- Cook HT. The origin of renal fibroblasts and progression of kidney disease. Am J Pathol. 2010; 176(1):22–24
- Hoitsma AJ, Woodle ES, Abramowicz D, Proot P, Vanrenterghem Y. FTY720 combined with tacrolimus in de novo renal transplantation: 1-year, multicenter, open-label randomized study. Nephrol Dial Transplant. 2011;26(11):3802–3805
- Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–360
- Peters H, Martini S, Wang Y, et al. Selective lymphocyte inhibition by FTY720 slows the progressive course of chronic anti-thy1 glomerulosclerosis. Kidney Int. 2004;6(4):1434–1443
- Awad AS, Ye H, Huang L, et al. Selective sphingosine 1-phosphate 1 receptor activation reduces Ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290(6):1516–1524
- Schaier M, Vorwalder S, Sommerer C, et al. Role of FTY720 on M1 and M2 macrophages, lymphocytes, and chemokines in 5/6 nephrectomized rats. Am J Physiol Renal Physiol. 2009;297(3):769–780
- Krämer S, Binder E, Loof T, et al. The lymphocyte migration inhibitor FTY720 attenuates experimental hypertensive nephropathy. Am J Physiol Renal Physiol. 2009;297(1):F218–F227
- Sui M, Zhou J, Xie R, et al. The sphingosine-1-phosphate receptor agonist FTY720 prevents the development of anti-glomerular basement membrane Glomerulonephritis. Mol Biol Rep. 2012;39(1):389–397
- Ji L, Masuda S, Saito H, Inui K. Down-regulation of rat organic cation transporter rOCT2 by 5/6 nephrectomy. Kidney Int 2002;62:514–524
- Finney CA, Hawkes CA, Kain DC, et al. S1P is associated with protection in human and experimental cerebral malarial. Mol Med. 2011;17(7–8):717–725
- Chen JF, Ni HF, Pan MM, et al. Pirfenidone inhibits macrophage infiltration in 5/6 nephrectomized rats. Am J Physiol Renal Physiol. 2013;304(6):F676–F685
- An WS, Kim HJ, Cho KH, Vaziri ND. Omega-3 fatty acid supplementation attenuates oxidative stress, inflammation, and tubulointerstitial fibrosis in the remnant kidney. Am J Physiol Renal Physiol. 2009;297(4):895–903
- Krämer S, Loof T, Martini S, et al. Mycophenolate mofetil slows progression in anti-thy1-induced chronic renal fibrosis, but is not additive to a high dose of enalapril. Am J Physiol Renal Physiol. 2005;289(2):F359–F368
- Anders HJ. Four danger response programs determine glomerular and tubulointerstitial kidney pathology: clotting, inflammation, epithelial and mesenchymal healing. Organogenesis. 2012;8(2):1–12
- Boor P, Sebeková K, Ostendorf T, Floege J. Treatment targets in renal fibrosis. Nephrol Dial Transplant. 2007;22(12):3391–3407
- Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010;21(11):1819–1834
- Anders HJ, Ryu M. Renal microenvironments and macrophage Phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011;80(9):915–925
- Tapmeier TT, Fearn A, Brown K, et al. Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney Int. 2010;78(4):351–362
- Husby G, Tung KS, Williams RC Jr. Characterization of renal tissue lymphocytes in patients with interstitial nephritis. Am J Med. 1981;70:31–38
- Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5(7):560–570
- Singer II, Tian M, Wickham LA, et al. Sphingosine-1-phosphate agonists increase macrophage homing, lymphocyte contacts, and endothelial junctional complex formation in murine lymph nodes. J Immunol. 2005;175(11):7151–7161
- Eardley KS, Zehnder D, Quinkler M, et al. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int. 2006;9(7):1189–1197
- Rovin BH, Lu L, Marsh CB. Lymphocytes induce monocyte chemoattractant protein1 production by renal cells after Fc gamma receptor cross-linking: role of IL-1 beta. J Leukoc Biol. 2001;69(3):435–439
- Ricardo SD, Van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118(11):3522–3530
- Lin SL, Castaño AP, Nowlin BT, Lupher Jr ML, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183(10):6733–6743
- Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14(11):1835–1842
- Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355(11):1124–1140
- Pfeilschifter W, Czech-Zechmeister B, Sujak M, Foerch C, Wichelhaus TA, Pfeilschifter J. Treatment with the immunomodulator FTY720 does not promote spontaneous bacterial infections after experimental stroke in mice. Exp Transl Stroke Med. 2011;3:2–7
- Johnson TA, Lapierre Y, BarOr A, Antel JP. Distinct properties of circulating CD8+ T cells in FTY720-treated patients with multiple sclerosis. Arch Neurol. 2010;67:1449–1455