Abstract
Objectives: Acute kidney injury (AKI) treated with continuous renal replacement therapy (CRRT) is associated with poor outcome. Plasma B-type natriuretic peptide (BNP) is a biomarker related to fluid volume overload, and is elevated in AKI patients. The purpose of the study was to assess whether BNP levels at the time of starting CRRT could be used as a predictor of mortality in patients with AKI receiving CRRT. Methods: We conducted a prospective observational cohort study enrolling 149 patients with AKI receiving CRRT. The primary outcome was mortality during CRRT. Results: The median BNP level of 84 (56.3%) patients who expired was significantly higher than that of those who survived (1812.5 vs. 475.0 pg/mL; p = 0.01). Receiver operating characteristic curves demonstrated BNP levels as a predictor of mortality during CRRT with an area under the curve of 0.77 (p = 0.000), and the optimal threshold for BNP was 1054 pg/mL. Patients with BNP levels above 1054 pg/mL had a significantly higher mortality (76.6 vs. 34.7%; p = 0.01). Conclusion: Elevated BNP level is associated with mortality in patients with AKI receiving CRRT.
Introduction
Acute kidney injury (AKI) is a relatively common complication and associated with sustained morbidity and mortality.Citation1–3 The incidence of severe AKI requiring renal replacement therapy (RRT) is 3–5%.Citation4 There are currently no specific pharmacological interventions for patients with established AKI, and RRT remains a key component of supportive care. Continuous renal replacement therapy (CRRT), which was introduced to expand the dialysis options for critically ill patients from traditional, intermittent hemodialysis (IHD) techniques, allows extracorporeal treatment with hypercatabolism and fluid overload.Citation5,Citation6 However, survival rates of those with sever AKI necessity RRT rarely exceed 50% despite the introduction of CRRT.Citation7,Citation8 Brain-type natriuretic peptide (BNP) is a member of the family of structurally related hormones—this natriuretic peptide is secreted from the ventricular myocardium in response to myocardial stretching and volume overload.Citation9,Citation10 BNP is useful in diagnosing or excluding heart failure as well as in estimating prognosis.Citation11,Citation12 BNP level is elevated in conditions such as sepsis, acute lung injury, pulmonary embolism and intracranial hemorrhage.Citation13–16
Previous investigations have reported that BNP levels in patients with sepsis, severe sepsis and septic shock correlate with their prognosis.Citation17,Citation18 However, few studies have focused on the significance of BNP in patients with severe AKI requiring CRRT, although such patients are expected to have over volume load, high cardiovascular events and mortality rates. The aim of this prospective study was to evaluate the BNP level to predict the clinical outcome of AKI undergoing CRRT.
Methods
Patients and data collection
This single center prospective observational study included 149 patients with AKI requiring CRRT at Dong-A university hospital from September 2010 to October 2012. AKI was defined as the RIFLE (Risk, Injury, Failure, Loss, and End-stage kidney disease) criteria.Citation19 The exclusion criteria included patients with end-stage renal disease (patients maintaining chronic dialysis), history of renal transplantation or medical history of chronic kidney disease (patients with a known decrease in glomerular filtration rate <15 mL/min/1.73 m2). The data including age, sex, medical information and vital signs were immediately recorded at the time of patient enrolment. Examinations such as blood routine study, arterial gas analysis and biochemical results were recorded at the time of starting CRRT. Disease severity was assessed using the Acute Physiology and Chronic Health Evaluation II (APAHCHE II) scores.Citation19 The criteria for organ failure and conception of sepsis have been proposed by previous reports.Citation20,Citation21 Survivors in this study were alive after finishing CRRT.
Laboratory and B-type natriuretic peptide measurements
The following laboratory studies were performed: complete blood cell count, serum electrolyte, serum chemistry for renal function tests and arterial blood gas analysis. Measurement of BNP levels was performed at the time of starting CRRT. Venous blood samples were collected and stored at minus 80 °C. Quantitative measurements were obtained by immunofluorescence labeling using a Triage-of-care analyzer (Biosite, San Diego, CA), with upper and lower limits of detection of 5000 pg/mL and 5 pg/mL, respectively.
Continuous renal replacement therapy
Indication for continuous renal replacement therapy (CRRT) included (1) azotemia (blood urea nitrogen >80 mg/dL and serum creatinine >3.0 mg/dL) and uremic symptoms, (2) fluid overload refractory to diuretics use with central venous pressure >14 mmHg, (3) hyperkalemia (>5.5 mEq/L) refractory to medical treatment, (4) oliguria (urine amount <200 mL/8 hour) refractory to diuretics and (5) metabolic acidosis (pH < 7.2 in arterial blood gas analysis). In the presence of multiple organ failure, CRRT was started earlier with an increasing serum creatinine level above 2.0 mg/dL or serum urea level above 60 mg/dL. Bedsides applications of continuous veno-venous hemodiafiltration (CVVHDF) using a CRRT instrument (Prisma, Prismaflex; Gambro Renal Products) with AN 69 high flux dialyzer (M100; Gambro Renal Products), we use the internal jugular and femoral veins for vascular access. The flow rate of the dialysate (Gambro; Na = 135 mEq/L, Ca = 0 mEq/L, K = 4.0 mEq/L, Cl = 112 mEq/L, NaHCO3 = 28 mEq/L, Mg = 1 mEq/L, dextrose = 200 g/L) was held at 800–1800 mL/hour, depending on clearance. Blood flow and ultrafiltration rates were adjusted at 80–150 mL/min and 30–50 mL/kg/hour according to patients’ requirements, respectively. A continuous infusion of nafamostat mesylate (10–40 mg/hour) into the circuit before the hemofilter was priming as the anticoagulant.
Statistical analysis
The primary end point was mortality during CRRT. Continuous data are presented as mean ± S.D. The non-parametric Mann–Whitney U test was used to compare numerical data between survivors and non-survivors. Fisher’s exact test was used to compare dichotomous data between survivors and non-survivors. The Mann–Whitney U test was used to compare numerical data and Fisher’s exact test was used to compare dichotomous data between the two groups according to BNP. To define an optimal decision threshold of BNP level for predicting mortality, a receiver operator characteristic plot analysis was performed. The Youden index (namely, sensitivity + specificity−1) was used to determine the optimal BNP threshold. Multivariate logistic regression analysis was used to test whether this threshold of BNP level was an independent predictor of mortality. p Values less than 0.05 were considered significant. All statistical calculations were performed with SPSS software, version 16.0 (SPSS Inc., Chicago, IL).
Results
During the study period, 182 patients required CRRT. Of these patients, 33 were excluded because of a history of CKD, dialysis, or recent renal transplantation. A total of 149 patients (81.9%) suffering from AKI receiving CRRT were recruited into this study. The etiologies of AKI were sepsis (79.9%), cardiac dysfunction (16.8%), hepatorenal syndrome (4.7%), rhabdomyolysis (2.7%), contrast nephropathy (1.3%) and drug-related nephropathy (0.7%). Sepsis was a contributing factor to AKI patients. The major primary sources of sepsis were thoracic in 41 patients (34.5%), intra-abdominal infection in 25 patients (21.0%), endovascular infection in 18 patients (15.1%), urogenital infection in 17 patients (14.2%) and unknown sources in 10 patients (8.4%). The indications for CRRT were as follows: oliguria in 132 patients (88.6%), azotemia in 72 patients (48.3%), fluid overload refractory to diuretics in 41 patients (27.5%), hyperkalemia in 14 patients (9%) and acidosis in 4 patients (2.7%). describes the baseline characteristics of the study population. The mean age was 60.9 ± 13.5 years in the survivor group and 63.9 ± 14.0 years in the non-survivor group; this age difference was not statistically significant. The prevalence of sepsis and oliguria was not significant between the two groups. The following factors were significantly different between survivors and non-survivors: ventilator care (55.4 vs. 85.7; p = 0.01), number of vasoactive drugs (1.2 ± 1.0 vs. 1.7 ± 1.1; p = 0.01), number of failed organs (2.3 ± 0.7 vs. 2.6 ± 0.7; p = 0.02), APACHE II scores (25.9 ± 6.9 vs. 29.5 ± 6.6; p = 0.01), systolic blood pressure (109.5 ± 18.6 vs. 101.6 ± 16.2 (mmHg); p = 0.01), diastolic blood pressure (70.3 ± 13.1 vs. 65.7 ± 12.6 (mmHg); p = 0.03), BNP levels (796.1 ± 897.6 vs. 2311.3 vs. 434.6 (pg/mL); p = 0.01), and procalcitonin (24.7 ± 36.2 vs. 52.1 ± 40.2 (ng/mL); p = 0.01).
Table 1. Baseline characteristics of the study population.
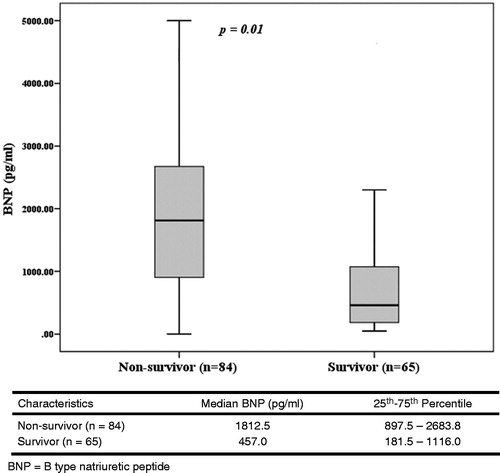
lists the characteristics of CRRT. The mean durations of treatment were similar between the two groups. Although the intensity of treatment (flow rate of effluent, 39.4 ± 6.27 vs. 37.6 ± 8.20 mL/kg/hour) was likely to be higher in the survivor group, there was no statistical difference between the two groups (p = 0.13). Eighty-four patients (56%) died during the course of CRRT. The median BNP level in patients who died was significantly higher than in those who survived (1812.5 vs. 457.0 pg/mL; p = 0.01; ). To assess the use of BNP measurements to predict mortality, a conventional receiver operator characteristic curve (ROC) was generated and the area under the curve (AUC) was calculated (). The AUC was 0.77 (95% confidence interval, 0.69–0.84), and the optimal threshold for BNP was 1054 pg/mL. At this cutoff, the sensitivity was 70.1%, specificity was 74.3%, positive predictive value was 76.6% and negative predictive value was 65.1%. depicts the characteristics of patients stratified according to BNP levels less than and more than the threshold of 1054 pg/mL. Patients in the above group were older, and had a higher number of vasoactive drugs and failed organs. A higher proportion of the above group was mechanically ventilated. The above group was associated with a significantly higher mortality as compared with the below group (76.6% vs. 34.7%; p = 0.01). No significant differences were found between the two groups in terms of time to start CRRT, duration of CRRT, dose of CRRT and net ultrafiltration. Multivariate analysis to determine the predictor of CRRT mortality was performed among covariates with p < 0.05 in the univariate analysis. Taking ventilator care, the number of vasoactive drug, number of failed organs, APACHE II scores, systolic BP, diastolic BP and BNP levels, and procalcitonin levels were entered into logistic regression analysis. summarizes the standardized regression coefficients with a 95% confidence interval and p-value for each factor. Only the BNP level was identified as a significant predictor of CRRT mortality.
Figure 2. Receiver operating characteristic curve for BNP at the start of CRRT. Area under the ROC curve was 0.77 and sensitivity and specificity were 71.2% and 74.8%, respectively.
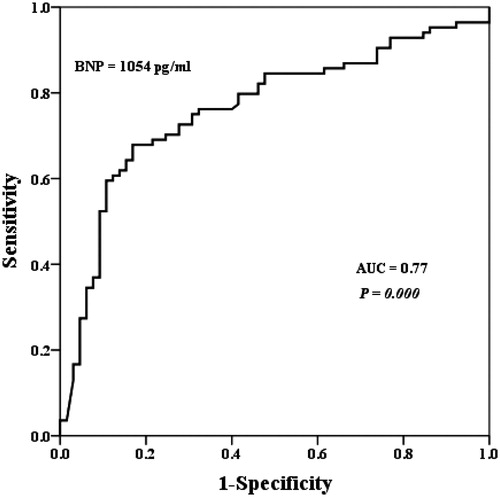
Table 2. Baseline characteristics of study treatments and use of CRRT.
Table 3. Patients’ characteristics stratifies according to BNP level upon starting CRRT.
Table 4. Multivariable logistic regression analysis for the prediction of mortality in patients during CRRT.
Discussion
The major finding of this study is that BNP at the time of starting CRRT is able to predict mortality in AKI patients receiving CRRT. In critically ill patients, continuous forms of renal replacement therapy are preferred to improve their cardiovascular tolerance.Citation22 Age, the need for artificial ventilation, the use of inotropics, urine volume, arterial base deficit and the number of failed organs have served as classical predictors of mortality during CRRT.Citation23,Citation24 The relative specific cardiac biomarker BNP afforded an additional impact on the mortality of patients with CRRT.
The major stimulus for BNP synthesis is increased LV wall stress,Citation25 which may occur as a result of volume expansion, pressure overload and increased wall tension.Citation26 In addition to increased mechanical wall stress that stimulates the release of myocardial BNP, there is recent evidence suggesting that ischemia and hypoxia may trigger BNP expression in the human myocardium independent of mechanical stress.Citation27 Volume management is an essential component in the maintenance of hemodynamic stability, tissue perfusion and organ function. In some conditions, such as sepsis, acute respiratory distress syndrome and surgical intensive care unit patients, volume overload increases morbidity and mortality.Citation28,Citation29 Patients with AKI also have an impaired ability to regulate volume management, so they tend to be in a volume overloaded state. Thus, circulating BNP levels may be elevated in AKI patients. In our study, BNP levels in AKI patients (1645.9 ± 340.8 pg/mL) were higher compared to those of young, healthy adults, 90% have BNP level < 25 pg/mL.Citation30 Limited data are available regarding the diagnostic and prognostic utility of BNP in patients with AKI requiring CRRT. In a recent study, de Cal et al. demonstrated that patients with AKI have higher levels of BNP compared to no AKI patients, and BNP levels of AKI patients continue to increase in the subsequent 48 hours.Citation31 Moreover, Fülöp et al.Citation32 found that volume-related weight gain is an important prognostic factor for survival in critically ill AKI patients treated with CRRT. With regard to mechanism of elevated BNP in AKI, using BNP levels in the assessment of volume overload may aid to predict prognosis in patients with AKI receiving CRRT. In this study, the plasma BNP level was determined as an independent predictor of mortality in patients with AKI receiving CRRT. The median BNP level of patients who expired during CRRT was significantly higher than that of survivors (1812 vs. 457.0 pg/mL, p = 0.01). BNP levels above the optimal discriminary threshold of 1054 pg/mL, determined by the ROC curve analysis were associated with the occurrence of mortality during CRRT (AUC: 0.77, p = 0.01).
A previous study has revealed that BNP levels were increased in patients with severe sepsis.Citation33 Critical illness like sepsis is associated with intense inflammatory syndrome characterized by markedly increased circulating proinflammatory cytokines. Selective upregulation of cardiac BNP occurs at the transcriptional and translational levels by proinflammatory cytokines.Citation34 In this study, the rate of sepsis was 83.1% in the survivor group and 77.4% in the non-survivor group (p = 0.39). APACHE II scores were higher in the BNP > 1054 pg/mL group (28.8 ± 6.8 vs. 26.9 ± 7.0), but did not show significant difference (p = 0.08).
Plasma BNP levels can be affected by other variable such as renal clearance. There is some suggestion that the glomerular filtration rate (GFR) has an independent confounding effect on increased BNP and BNP levels with deteriorating renal function.Citation35,Citation36 Another study of non-dialysis chronic kidney disease (CKD) patients suggested that GFR was a more important determinant of serum BNP than LV function.Citation37 Conversely, Takami et al. showed that even though BNP was correlated with renal function, the markers of LV overload (including LV end-diastolic volume and pressure) remain important determinants of plasma BNP level independent of renal function.Citation38 In our study, the mean GFR of the total patient population was 22.1 ± 1.1. The value was 21.1 ± 11.0 in the BNP above 1054 pg/mL group and 23.1 ± 14.7 in the BNP below 1054 pg/mL group; this difference was not statistically significant (p = 0.33). There are several limitations to this study. First, BNP levels more than 1054 pg/mL are independent markers of mortality during CRRT. However, with a positive predictive value of 76.7%, and the AUC was 0.77, demonstrating that the clinical implications of this association may be limited and should be interpreted with caution. Second, we have just one measurement of BNP levels at the time of starting CRRT. Change in BNP level during intensive care unit (ICU) stay or BNP level at discharge, which may reflect the effectiveness of ICU treatment and be associated with patient outcome.Citation39 However, the design of this study included one measurement of BNP at the beginning of CRRT to respond to the question: is the BNP level at the start of CRRT predictive marker of mortality during CRRT? Third, we did not perform objective assessment of cardiac function to document that increased BNP in this setting would be due to myocardial dysfunction. Fourth, this study included a relative small number of patients. However, most studies evaluating natriuretic peptide as a prognostic marker in critically ill patients have been of similar size.
In conclusion, we showed that BNP levels in patients with AKI receiving CRRT were associated with mortality. A BNP level of more than 1054 pg/mL is an independent marker of short-term mortality. Further prospective studies with larger cohorts are needed to evaluate the optimal threshold and independent predictive power of BNP in patients requiring CRRT.
Declaration of interest
Authors have nothing to declare.
Acknowledgements
This study was supported by research funds from Dong-A University.
References
- Ahlstrom A, Tallgren M, Peltonens S, Rasanen P, Pettila V. Survival and quality of life of patients requiring acute renal replacement therapy. Intensive Care Med. 2005;31(9):1222–1228
- Bagshaw SM, Laupland KB, Doig CJ, et al. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9(6):R700–R709
- Korkeila M, Ruokonen E, Takala J. Costs of care, long-term prognosis and quality of life in patients requiring renal replacement therapy during intensive care. Intensive Care Med. 2000;26(12):1824–1831
- Uchinos S. The epidemiology of acute renal failure in the world. Curr Opin Crit Care. 2006;12(6):538–543
- Bellomo R, Mehta R. Acute renal replacement in the intensive care unit: now and tomorrow. New Horiz. 1995;3(4):760–767
- Canaud B, Mion C. Extracorporeal treatment of acute renal failure: methods, indications, quantified and personalized therapeutic approach. Adv Nephrol Necker Hosp. 1995;24:271–313
- Barton IK, Hilton PJ, Taub NA, et al. Acute renal failure treated by hemofiltration: factors affecting outcome. Q J Med. 1993;86(2):81–90
- Sasaki S, Gando S, Kobayashi S, et al. Predictors of mortality in patients treated with continuous hemofiltration for acute renal failure in an intensive care setting. ASAIO. 2001;47(1):86–91
- de Denus S, Pharand C, Williamson DR. Brain natriuretic peptide in the management of heart failure: the versatile neurohormone. Chest. 2004;125(2):652–668
- Azzazy HM, Christenson RH. B-type natriuretic peptide: physiologic role and assay characteristics. Heart Fail Rev. 2003;8(4):315–320
- Januzzi JrJL, Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95(8):948–954
- Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161–167
- Rudiger A, Gasser S, Fischler MN, Hornemann T, von Eckardstein A, Maggiorini M. Comparable increase of B-type natriuretic peptide and amino-terminal pro-B-type natriuretic peptide levels in patients with severe sepsis, septic shock, and acute heart failure. Crit Care Med. 2006;34(8):2140–2144
- Rana R, Vlahakis NE, Daniels CE Rana R, Vlahakis NE, Daniels C. B-type natriuretic peptide in the assessment of acute lung injury and cardiogenic pulmonary edema. Crit Care Med. 2006;34(7):1941–1946
- Lega JC, Lacasse Y, Lakhal L, Provencher S. Natriuretic peptides and troponins in pulmonary embolisms: a meta-analysis. Thorax. 2009;64(10):869–875
- James ML, Blessing R, Phillips-Bute BG, Bennett E, Laskowitz DT. S100B and brain natriuretic peptide predict functional neurological outcome after intracerebral hemorrhage. Biomarkers. 2009;14(6):388–394
- Rivers EP, McCord J, Otero R, Jacobsen G, Loomba M. Clinical utility of B-type natriuretic peptide in early severe sepsis and septic shock. J Intensive Care Med. 2007;22(6):363–373
- Hoffmann U, Brueckmann M, Bertsch T, et al. Increased plasma levels of NT-proANP and NT-proBNP as markers of cardiac dysfunction in septic patients. Clin Lab. 2005;51(7–8):373–379
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the second international cocsensus conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):204–212
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. Prognosis in acute organ system failure. Ann Surg. 1985;202(6):685–693
- Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis definitions Conference. Crit Care Med. 2003;31(4):1250–1256
- Prowle JR, Bellomo R. Continuous renal replacement therapy: recent advancement and future research. Nat Rev Nephrol. 2010;6(9):521–529
- Schwilk B, Wiedeck H, Stein B, reinelt H, Treber H, Bothner U. Epidemiology of acute renal failure and outcome of hemodiafiltration in intensive care. Intensive Care Med. 1997;23(12):1204–1211
- Baudouin SV, Wiggins J, Keogh BF, Morgan CJ, Evans TW. Continuous veno-venous hemofiltration following cardio-pulmonary bypass. Indications and outcome in 35 patients. Intensive Care Med. 1993;19(5):290–293
- Kinnunen P, Vuolteenaho O, Ruskoaho H. Mechanisms of arterial and brain natriuretic peptide release from rat ventricular myocardium: effect of stretching. Endocrinology. 1993;132(5):1961–1970
- Tokola H, Hautala N, Marttila M, et al. Mechanical load-induced alterations in B-type natriuretic peptide gene expression. Can J Physiol Pharmacol. 2001;79(8):646–653
- Mollamach F, Zoccali C, Tripepi G, et al. Diagnostic potential of cardiac natriuretic peptides in dialysis patients. Kidney Int. 2001;59(4):1559–1566
- Sakr Y, Vincent JL, Reinhart K, et al. Sepsis Occurrence in Acute Ill Patients Investigations. High tidal volume and positive fluid balance are associated with worse outcome in acute lung injury. Chest. 2005;128(5):3098–3108
- Vincent JL, Sakr Y, Sprung CL, et al. Sepsis Occurrence in Acute Ill patients Investigators. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–353
- Daniels LB, Allison MA, Clopton P, et al. Use of natriuretic peptides in pre-participation screening of college athletes. Int J Cardiol. 2008;124(3):411–414
- de Cal M, Haapio M, Cruz DN, et al. B-type natriuretic peptide in the critically ill with acute kidney injury. Int J Nephrol. 2011;951629:1–6
- Fulop T, Pathak MB, Schmidt DW, et al. Volume-Related weight gain and subsequent mortality in acute renal failure patients treated with continuous renal replacement therapy. ASAIO J. 2010;56(4):333–337
- McLean AS, Huang SJ, Hyams S, et al. Prognostic value of B-type natriuretic peptide in severe sepsis and septic shock. Crit Care Med. 2007;35(4):1019–1026
- Ma KK, Ogawa T, de Bold AJ. Selective upregulation of cardiac brain natriuretic peptide at the transcriptional and translational levels by pro-inflammatory cytokines and by conditioned medium derived from mixed lymphocyte reactions via p38 MAP kinase. J Mol Cell Cardiol. 2004;36(4):505–513
- DeFilippi CR, Fink JC, Nass CM, Cher H, Christenson R. N-terminal pro-B-type natriuretic peptide for predicting coronary disease and left ventricular hypertrophy in asymptomatic CKD not requiring dialysis. Am J Kidney Dis. 2005;46:35–44
- Khan IA, Fink J, Nass C, Chen H, Christenson R, de Filippi CR. N-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide for identifying coronary artery disease and left ventricular hypertrophy in ambulatory chronic kidney disease patients. Am J Cardiol. 2006;97(10):1530–1534
- Mark PB, Stewart GA, Gansevoort RT, et al. Diagnostic potential of circulating natriuretic peptides in chronic kidney disease. Nephrol Dial Tranplant. 2006;21(2):402–410
- Takami Y, Horio T, Iwashima Y, et al. Diagnostic and prognostic value of plasma brain natriuretic peptide in non-dialysis-dependent CRF. Am J Kidney Dis. 2004;44(3):420–428
- Cheng V, Kazanagra R, Garcia A, et al. A rapid bedside test for B-type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: a pilot study. J Am Coll Cardiol. 2001;37(2):386–391