Abstract
The purpose of this study was to determine whether toll-like receptors 9 (TLR9) gene polymorphisms (rs352139 and rs352140) were markers of susceptibility to the development and progression of membranous nephropathy (MGN) in Taiwanese patients. The polymorphisms were investigated by polymerase chain reaction in 397 Taiwanese individuals (134 MGN patients and 263 controls). Patients with malignancy, chronic infectious diseases, lupus nephritis, or drug-induced secondary MGN were excluded from the study. Data showed AA genotype at rs352139 SNP or GG genotype at rs352140 SNP may indicate higher risk for MGN (odds ratio [OR] = 1.55; 95% confidence interval [CI] = 1.02–2.35, at rs352139 SNP; OR = 1.57; 95% CI = 1.03–2.39, at rs352140 SNP). However, MGN patients with A–G haplotype were susceptible for decreased creatinine clearance rate and for seriously tubule-interstitial fibrosis. The result suggests for the first time that TLR9 (rs352139 and rs352140) polymorphisms may contribute to the development and progression of MGN.
Introduction
Membranous glomerulonephritis (MGN) is an immune-mediated glomerular disease characterized by abundant, nonselective proteinuria, and a variable clinical course and prognosis.Citation1 Although spontaneous remission may occur in some cases and several treatment options are now available, a significant number of patients have poor response to treatment and their risks of kidney disease progression remain high.Citation2 Until recently, autoantibodies of the IgG4 subclass to at least three podocyte membrane proteins have been identified, including the phospholipase A2 receptor, manganese superoxide dismutase, aldose reductase in small cohorts of patients.Citation3–6 Although it is clear that autoantibodies to podocyte membrane proteins are elicited in idiopathic MN, questions remain concerning the triggers for the development and the mechanisms for the progression of the disease. Immune complexes can be isolated in MN, implicating the involvement of an inflammatory process in this disease.Citation7 Further evidence that this disease is mediated by immune complexes is provided by the presence of immunoglobulins and complement components in subepithelial capillary walls, as well as the morphologic and immunopathologic similarities between experimental MGN and immunological glomerular diseases.Citation7 The deposits of these immunoglobulins observed in MGN may develop from circulating immune complexes, or because of in situ formation or previous deposits of foreign antigens.Citation8 Furthermore, the pathogenesis of MGN-mediated interstitial inflammation and fibrosis remains unclear, although renal function and the course of MGN are more strongly correlated with the degree of tubulointerstitial damage than the extent of the glomerular lesions.Citation9,Citation10 Therefore, although MGN is a multi-factorial disease, the inflammatory pathway may play an important role in its pathogenesis.Citation3,Citation7
MGN is the most common primary cause of nephrotic syndrome, accounting for approximately 40% of cases in adults.Citation11 It is characterized by basement membrane thickening and subepithelial immune deposits, without cellular proliferation or infiltration.Citation2 In approximately 25% of the cases, MGN appears secondary to other conditions, such as infections, neoplasms, and systemic lupus erythematosus (SLE).Citation12 MGN may also lead to the development of chronic kidney disease (CKD), and finally result in end-stage renal disease (ESRD).Citation13 That MGN may be one of the causes of ESRD is of particular importance in Taiwan, which has the highest prevalence of this disease in the world.Citation14–16 The study of inflammatory factors associated with MGN will therefore be helpful in further understanding this disease and preventing the development of ESRD.
Toll-like receptors (TLRs) play a central role in the response of both the innate and the adaptive immune system to microbial ligands.Citation17 It is realized now that viral infections turn non-plasmacytoid dendritic cells (non-pDCs) into high IFN-α producers in a TLR-dependent process.Citation18 Plasmacytoid dendritic cells (pDCs) exhibit TLR3 and TLR7 for viruses and TLR9 for the CpG-DNA of bacteria. Tonsil B cells also express TLR9 and TLR10 for microbial products. The oligo deoxy-nucleotide CpG-DNA induces pDCs to produce IFN-α and IP-10, IFN-inducible protein. CpG-DNA will activate B lymphocytes.Citation19,Citation20 Glomerular disease can be triggered or exacerbated by microbes that activate the immune system by Toll-like receptor (TLR) ligation.Citation21,Citation22 Exaggerated activation of TLRs is associated with ischemic kidney damage, acute kidney injury, end-stage renal failure, acute tubulointerstitial nephritis, acute renal transplant rejection, and delayed allograft function.Citation22 TLR9 have been implicated in the initiation and disease progression of several forms of human and experimental kidney disease, such as lupus nephritis, IgA nephropathy, and crescentic glomerulonephritis.Citation23–25 However, no genetic study examining the relationship of TLR9 gene polymorphisms to MGN disease has been carried out to date.
As the present study aimed to identify genetic polymorphisms in potential candidate genes implicated in MGN, the association of TLR9 gene polymorphisms with MGN in a Taiwanese population was investigated. Our examination involved a retrospective review of the pathology reports of all native renal biopsies performed at the Taichung Veterans General Hospital during 1982–2008. Our findings are expected to further elucidate the role of TLR9 gene polymorphisms in the progression of MGN disease, with a view to identify possible management strategies for this common nephropathy.
Materials and methods
Study population
From 1982 to 2008, 134 patients with previously diagnosed renal biopsy-approved membranous glomerulonephritis (MGN) and 263 gender-age-matched unrelated healthy adults to serve as a control group were recruited from the Taichung Veterans General Hospital and China Medical University Hospital. Patients with malignancy, chronic infectious diseases (including infections with hepatitis B and C viruses), lupus nephritis, or drug-induced secondary MGN were excluded from the study. The general demographic data (gender, body weight, systolic/diastolic pressure, and body height) and medical information (duration of follow-up, occurrence of renal failure, use of herbal substances, etc.) of all patients were reviewed. Patient characteristics included demographic variables, clinical and laboratory data obtained over the course of the disease, the occurrence of vascular events (cardiovascular disease and peripheral vascular events), the treatment regimens used, and the responses to these regimens. All participants gave their written informed consent for participation prior to commencement of the study. The study was approved by the institutional review board (No. C08159) of the Taichung Veterans General Hospital.
The selection of treatment modality, either supportive or aggressive with immunosuppressants, was based on the treating physician’s decision. Supportive therapy depended on a patient’s symptoms, and usually included diuretics, angiotensin-converting enzyme inhibitors (ACEIs), and/or angiotensin II receptor blockers (ARBs). The immunosuppressive therapies used include the following regimens: (a) only prednisolone, 1 mg kg−1 d−1; (b) a six-month course of corticosteroids alternating every other month with either chlorambucil at a dose of 0.2 mg kg−1 d−1 or cyclophosphamide at a dose of 1.5–2.0 mg kg−1 d−1; and (c) cyclosporine A (CyA, Neoral, Novatis) given at a dose of 3–5 mg kg−1 d−1 with or without prednisolone.
Response and outcomes
The responses to therapy were defined as follows: (a) no response; (b) partial remission as evidenced by a reduction of protein levels by more than 50% or a final protein level of 0.2–2.0 g d−1; or (c) complete remission, where proteinuria was less than 0.2 g d−1. The “progression of renal disease” was defined as doubling of baseline serum creatinine (Cr) values or the development of ESRD. A patient was considered to have developed ESRD when he/she required renal replacement therapy.
Renal biopsy review
Histological staging was based on histological lesions, including glomerular lesions, tubulointerstitial lesions, focal glomerulosclerosis, and fibrointimal lesions. Biopsy specimens were reviewed by a nephropathologist who was unaware of the clinical history, renal function, and TLR9 gene SNP (rs352139 and rs352140) status of the patients. A semiquantitative scoring system using a scale of 0 (absent), 1 (mild: <25%), and 2 (moderate to severe: >25%) was employed for the assessment of tubulointerstitial changes and glomerular sclerosis/obsolescence under light microscopy. Staging of the disease was determined according to the findings obtained using electron microscopy.
SNP selection
TLR9 SNP genotype information was downloaded from the HapMap CHB + JPT population in December 2008. HapMap genotypes were analyzed within Haploview, and Tag SNPs were selected using the Tagger function and by applying the following additional criteria: (i) a threshold minor allele frequency (MAF) in the HapMap CHB + JPT population of 0.05 for “tag SNPs”; and (ii) the availability of probe/primers that pass the criteria recommended by the manufacturer (ABI: Applied Biosystems Inc., Foster City, CA), in order to ensure a high genotyping success rate. The two polymorphisms that met the criteria, SNP rs352139 (A/G), located in intron 1, and SNP rs352140 (G/A), located in exon 2, were selected for further study.
Genomic DNA extraction and genotyping of TLR9 genetic polymorphisms
Genomic DNA was extracted from peripheral blood leukocytes according to standard protocols (Genomic DNA kit; Roche, Indianapolis, IN). The genotypes of our two selected TLR9 SNPs, rs352139 at chromosome position 52198412 (intron 1) and rs352140 at chromosome position 52196737 (exon 2), were determined using the TaqMan SNP genotyping assay (ABI). The primers and probes used to detect the SNPs were from the ABI assay on demand (AOD) kit, and reactions were carried out according to the manufacturer’s protocol. Briefly, polymerase chain reaction (PCR) was performed in the presence of 2× TaqMan® Universal PCR Master Mix (ABI), assay mix (Assay ID C__2301953_10 and C__2301954_10; ABI), and genomic DNA (15 ng). After an initial denaturation for 10 min at 95 °C, 40 cycles consisting of denaturation at 95 °C for 15 s, and annealing at 60 °C for 60 s were run. The probe fluorescence signal detection was performed using the ABI Prism 7900 Real Time PCR System.
Statistical analysis
Chi-square tests or Fisher’s exact tests were used to identify statistically significant differences in allele/genotype frequencies between case and control groups. Allelic frequencies were expressed as a percentage of the total alleles. The Hardy–Weinberg equilibrium was tested for each marker using a χ2-test. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were derived by logistic regressions to correlate TLR9 alleles/genotypes/haplotypes with MGN susceptibility. The Kaplan–Meier method was used to estimate cumulative survival, while differences in survival were analyzed with the log-rank test. All data were analyzed with the SPSS Version 15.0 software (SPSS Inc., Chicago, IL). A p value <0.05 was considered statistically significant.
Results
gives the genotypic and allelic frequencies of rs352139 and rs352140, with genotype distributions in accordance with the Hardy–Weinberg equilibrium. The A allele was the most commonly detected allele at the rs352139 polymorphism, both in MGN patients (69.0%; 185/268) and controls (64.3%; 341/530), while the G allele was most commonly detected at the rs352140 polymorphism in MGN patients (70.1%; 188/268) and controls (65.2%; 343/526). When the distribution of genotype frequencies was compared between MGN patient and control groups, statistically significant differences were noted for both the rs352139 and rs352140 SNPs in MGN patients and controls (p = 0.040 and 0.034, respectively). Our data indicates that individuals with the AA genotype at rs352139 SNP and/or the GG genotype at rs352140 SNP may have a higher risk of developing MGN (odds ratio [OR] = 1.55 and 1.57, respectively; 95% confidence interval [CI] = 1.02–2.35 and 1.03–2.39, respectively) ().
Table 1. Genotypic and allelic frequencies of TLR9 genetic polymorphisms in MGN patients and controls.
Haplotype frequencies were estimated using the rs7744, rs352140, and rs352139 SNPs (). Two haplotypes of TLR9 were present in our study population: A–G and G–A was the common haplotypes found in both MGN patients (68.7% and 29.5%, respectively) and the control group (64.0% and 35.5%, respectively) ().
Table 2. Distribution of TLR9 haplotype frequencies in MGN patients and controls.
A comparison of the clinical features of MGN patients with and without the major A–G haplotype is shown in . No differences in gender distribution, age of onset, duration of follow-up, body mass index (BMI), mean blood pressure (MBP), or incidence of hematuria or proteinuria were observed. However, the baseline creatinine clearance (CCr) level detected in the initial laboratory test was found to be 83.6 ± 40.8 mL/min in MGN patients with the A–G haplotype and 63.1 ± 35.8 mL/min in those with the non-A–G haplotype (p = 0.034), although initial laboratory tests did not detect significant differences in baseline serum creatinine levels (Cr) or daily urinary protein excretion (DUP). Similarly, no significant differences in the data obtained in the final laboratory test, performed after a mean follow-up period of 12.9 ± 6.2 years, were observed. In addition, the decreased level of Ccr in MGN patients with A–G and non-A–G haplotype around 30.4% (from 83.6 ± 40.8 to 58.2 ± 42.1) and 23.6% (from 83.6 ± 40.8 to 58.2 ± 42.1), respectively.
Table 3. Comparison of the clinical features of MGN patients with and without the major A–G haplotype.
The relationship between the TLR9 haplotype and the pathological features of MGN was also analyzed. The scoring for MGN was performed using electron microscopy images of the glomeruli; however, only 107 biopsy specimens were available for review and scoring by the pathologist. Of these, 27 glomeruli (25.2%) at stage I, 55 (51.4%) at stage II, 18 (16.8%) at stage III, 5 (4.7%) at stage IV, and 2 (1.9%) at stage V were identified. As shown in , there were no differences in the results of histological examination, the percentage of global sclerosis, or the fibrointimal atherosclerosis score between the 2 haplotypes of MGN patients. However, when the incidence of the pathological features of tubule-interstitial fibrosis in MGN patients with different haplotypes was compared, we noted that MGN patients with A–G haplotype (9.2%, 9/98) had higher incidence of seriously tubule-interstitial fibrosis, compared with MGN patients with non-A–G haplotype 5.3%, 1/19) (). This suggests that the A–G haplotype may be a “risk” haplotype in the development of seriously tubule-interstitial fibrosis in MGN patients (p = 0.019).
Table 4. Comparison of the pathological features of MGN patients with and without the major A–G haplotype.
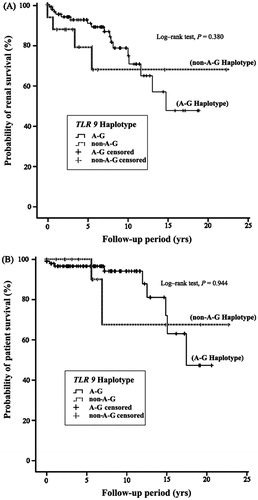
A log-rank test was used for survival analysis of MGN patients with and without the A–G haplotype of the TLR9 gene. As shown in , renal failure was observed in 42 patients during the follow-up period, with an incidence of 15.79% (42/266). Median and mean renal survival durations were 8.0 and 5.7 years, respectively. In total, 24 patients died during the follow-up period, and the mortality rate for our study was 9.02% (24/266). Median and mean survival durations were 9.6 and 12.9 years, respectively. The longest follow-up period for patients who died before the end-point of this study was 17.4 years, while the longest period for surviving patients was 22.7 years (). The Kaplan–Meier curves for renal disease and patient survival show that there are no statistically significant differences in survival between the A–G and non-A–G haplotypes of the TLR9 gene in MGN patients ().
Discussion
Currently, MGN is considered to be an autoimmune disease with immunologic expression that occurs in genetically susceptible individuals.Citation26 As such, the polymorphic gene sequences of cytokines known to be involved in the pathogenesis of MGN are potential markers of disease susceptibility. Previous studies have examined the relationship between the incidence of MGN and cytokine gene polymorphisms, including TNF-α gene G-308A, angiotensin-converting enzyme insertion/deletion [ACE I/D], angiotensin II receptor 1 (AT1R 1166A/C), angiotensinogen (AGT M235T), and NOS (ecNOS4b/a).Citation27–29 In our study, we focused on variants of the TLR9 gene (rs352139 and rs352140) that have previously been associated with SLE.Citation30
Our study has demonstrated a statistically significant association between MGN and the rs352139 and rs352140 polymorphisms of TLR9, whereby the frequencies of the AA genotype at rs352139 and the GG genotype at rs352140 were significantly higher in MGN patients than in control participants. It is estimated from our results that the A–G haplotype of the TLR9 gene is present in approximately 68.7% of MGN patients. Compared with the control group, the A–G haplotype appears to be a susceptibility factor for the development of MGN in our Taiwanese cohort, although the difference was not statistically significant (). On the other hand, we observed MGN patients with A–G haplotype accompanied worse situations both in patient survival and renal survival (), even data with no statistically significant. And these results may be due to (a) the higher level of clinical features including BMI, cholesterol, and triglyceride; (b) the seriously decreased of Ccr (around 30.4%; from 83.6 ± 40.8 to 58.2 ± 42.1); (c) about two folds patients with seriously tubule-interstitial fibrosis in MGN patients with A–G haplotype ( and ). According to our data, TLR9 A–G haplotype seem as a “risk factor” for MGN development and progression.
In addition, the sample size for MGN patients was indeed the major limitation of this study. Recruiting a sufficient number of the patients is indispensable not only for reducing false-positive results but also for increasing statistical power. We hope that in the near future, we can undertake a multicenter collaboration, which will allow us to increase the sample size of the study cohorts and to validate the association in multiple sample sets.
TLR9 expression levels have recently been proposed to influence the development of lupus nephritis, crescentic GN and IgA nephropathy;Citation22–25 however, no data are available concerning the genetic polymorphisms and expression of TLR9 in MGN patients. As rs352139 was found in our study to be an intronic SNP, it is possible that the expression level of TLR9 may be influenced by mRNA splicing. In addition, rs352140 corresponds to Pro545, which is located in a leucine-rich repeat domain. Because leucine-rich repeats are critical for TLR9 signal transduction, and proline mutations are often detrimental to protein folding, this could be a further mechanism by which this SNP impacts MGN. Hence, further studies are required to determine whether the polymorphisms identified in this study are associated with TLR9 expression levels in MGN patients.
As the patients in this study were recruited from only one center in Taiwan, the interpretation of our results is limited. However, our study findings suggest a significant role of TLR9 polymorphisms in the risk of developing MGN in a Taiwanese population. The identification of TLR9 polymorphisms as genetic risk factors for MGN susceptibility in Taiwan suggests that it may be beneficial to evaluate the use of these polymorphisms as prognostic markers in predictive clinical testing for MGN worldwide, particularly in ethnically disparate populations.
In conclusion, to the best of our knowledge, this is the first report examining TLR9 polymorphisms in MGN patients, and has for the first time demonstrated differential genotype distribution of the TLR9 gene between normal controls and MGN patients. Our findings suggest that the TLR9 gene, which is an important inflammation-related gene, may be associated with the development and renal deterioration in MGN patients.
Declaration of interest
No competing financial interests exist.
This work is supported in part by Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH101-TD-B-111-004), China Medical University (CMU100-S-06), China Medical University Hospital (DMR-101-041), and Asia University (CMU-asia-05) in Taiwan.
Y. T. Chen’s fellowship is supported by China Medical University (CMU101-AWARD-01), Taiwan.
Acknowledgments
We acknowledge Ms Hsuan-Min Chuang and Ms Chu-Cheng Tsai for their assistance with DNA extraction and genotype analysis.
References
- Schieppati A, Mosconi L, Perna A, et al. Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med. 1993;329:85–89
- Ponticelli C. Membranous nephropathy. J Nephrol. 2007;20:268–287
- Murtas C, Ravani P, Ghiggeri GM. New insights into membranous glomerulo-nephritis: from bench to bedside. Nephrol Dial Transplant. 2011;26:2428–2430
- Beck LH Jr, Bonegio RG, Lambeau G. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21
- Prunotto M, Carnevali ML, Candiano G. Autoimmunity in membranous nephron-pathy targets aldose reductase and SOD2. J Am Soc Nephrol. 2010;21:507–519
- Bruschi M, Carnevali ML, Murtas C. Direct characterization of target podocyte antigens and autoantibodies in human membranous glomerulonephritis: alpha-enolase and borderline antigens. J Prot. 2008;74:2008–2017
- Couser WG, Nangaku M. Cellular and molecular biology of membranous nephron apathy. J Nephrol. 2006;19:699–705
- Ronco P, Debiec H. New insights into the pathogenesis of membranous glomerulonephritis. Curr Opin Nephrol Hypertens. 2006;15:258–263
- Wehrmann M, Bohle A, Bogenschutz O, et al. Long-term prognosis of chronic idiopathic membranous glomerulonephritis. An analysis of 334 cases with particular regard to tubulointerstitial change. Clin Nephrol. 1989;31:67–76
- Troyanov S, Roasio L, Pandes M, et al. Renal pathology in idiopathic membranous nephropathy: a new perspective. Kidney Int. 2006;69:1641–1648
- Kodner C. Nephrotic syndrome in adults: diagnosis and management. Am Fam Physician. 2009;80:1129–1134
- Glassock RJ. Secondary membranous glomerulonephritis. Nephrol Dial Transplant. 1992;7:64–71
- Philibert D, Cattran D. Remission of proteinuria in primary glomerulonephritis: we know the goal but do we know the price? Nat Clin Pract Nephrol. 2008;4:550–559
- Maisonneuve P, Agodoa L, Gellert R, et al. Distribution of primary renal diseases leading to end-stage renal failure in the United States, Europe, and Australia/New Zealand: results from an international comparative study. Am J Kidney Dis. 2000;35:157–165
- Hsu CC, Hwang SJ, Wen CP, et al. High prevalence and low awareness of CKD in Taiwan: a study on the relationship between serum creatinine and awareness from a nationally representative survey. Am J Kidney Dis. 2006;48:727–738
- Grassmann A, Gioberge S, Moeller S, et al. ESRD patients in 2004: global overview of patient numbers, treatment modalities and associated trends. Nephrol Dial Transplant. 2005;20:2587–2593
- Medzhitov R, Janeway CJ. The Toll receptor family and microbial recognition. Trends Microbiol. 2008;8:452–456
- Diebold SS, Matoya M, Unger H, et al. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 2003;424:324–328
- Blackwell SE, Krieg AM. CpG-DNA induced monocyte IFN inducible protein 10 production is regulated by plasmacytoid DC derived IFN alpha. J Immunol. 2003;170:4061–4068
- Verthelyi D, Zeuner RA. Differential signaling by CpG DNA in DCs and B cells: not just TLR9. Trends Immunol. 2003;24:519–523
- Shirali AC, Goldstein DR. Tracking the toll of kidney disease. J Am Soc Nephrol. 2008;19:1444–1450
- Gluba A, Banach M, Hannam S, et al. The role of Toll-like receptors in renal diseases. Nat Rev Nephrol. 2010;6:224–235
- Pawar RD, Ramanjaneyulu A, Kulkarni OP, et al. Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. J Am Soc Nephrol. 2007;18:1721–1731
- Papadimitraki ED, Tzardi M, Bertsias G, et al. Glomerular expression of toll-like receptor-9 in lupus nephritis but not in normal kidneys: implications for the amplification of the inflammatory response. Lupus. 2009;18:831–835
- Summers SA, Steinmetz OM, Ooi JD, et al. Toll-like receptor 9 enhances nephritogenic immunity and glomerular leukocyte recruitment, exacerbating experimental crescentic glomerulonephritis. Am J Pathol. 2010;177:2234–2244
- Cattran DC. Idiopathic membranous glomerulonephritis. Kidney Int. 2001;59:1983–1994
- Chen CH, Shu KH, Wen MC, et al. Impact of plasminogen activator inhibitor-1 gene polymorphisms on primary membranous nephropathy. Nephrol Dial Transplant. 2008;23:3166–3173
- Bantis C, Heering PJ, Aker S, et al. Tumor necrosis factor-alpha gene G-308A polymorphism is a risk factor for the development of membranous glomerulonephritis. Am J Nephrol. 2006;26:12–15
- Stratta P, Bermond F, Guarrera S, et al. Interaction between gene polymorphisms of nitric oxide synthase and renin–angiotensin system in the progression of membranous glomerulonephritis. Nephrol Dial Transplant. 2004;19:587–595
- Xu CJ, Zhang WH, Pan HF, et al. Association study of a single nucleotide polymorphism in the exon 2 region of toll-like receptor 9 (TLR9) gene with susceptibility to systemic lupus erythematosus among Chinese. Mol Biol Rep. 2009;36:2245–2248