Abstract
Background: Studies detected an association between visfatin and markers of iron metabolism in patients with insulin resistance. In this study, such a relation was evaluated in hemodialysis (HD) patients. Also relations between visfatin and hepcidin, demands for recombinant human erythropoietin (rHuEpo), inflammation, and situations characterized by insulin resistance were evaluated. Methods: After a four-week washout period from iron treatment, 33 HD patients and 20 healthy volunteers enrolled in the study. Serum visfatin, hepcidin, and interleukin-6 (IL-6) were assessed by means of enzyme-linked immunosorbent assay. Hemoglobin, serum iron, ferritin, and transferrin saturation (TSAT) were also measured. Results: Visfatin was markedly increased in HD patients. Visfatin levels did not differ between diabetics and non-diabetics. No relation was detected between visfatin and body mass index or IL-6 in HD patients. From the markers of iron metabolism, the hepcidin included, visfatin was related only to TSAT. A strong positive relation was revealed between visfatin and hemoglobin, whereas visfatin was inversely related to rHuEpo dose. Resistance to rHuEpo index was inversely and independently of TSAT related to visfatin. Conclusion: Visfatin is increased in HD patients and it is associated with decreased demands for rHuEpo.
Introduction
Visfatin is considered as an adipokine, which is increased in case of insulin resistance and/or inflammation.Citation1,Citation2 At first, visfatin was identified as a cytokine that promotes B-lymphocyte maturation and named pre B-cell colony enhancing factor (PBEF).Citation3 Intracellularly it acts as an enzyme involved in nicotinamide adenine dinucleotide (NAD) salvage pathway and named nicotinamide phosphoribosyl transferase (Nampt).Citation4 Finally, visfatin was identified as an adipokine with insulin mimetic properties.Citation5
As noted, visfatin is increased in case of insulin resistance and/or inflammation,Citation1,Citation2 which are common in hemodialysis (HD) patients.Citation6,Citation7 Several studies have shown elevated serum visfatin level in HD and/or associations with metabolic parameters, atherosclerosis, and inflammation.Citation8–13
In patients with insulin resistance, iron metabolism is disturbed.Citation14 Iron metabolism is also disturbed in HD patients.Citation15 In patients with altered glucose tolerance serum visfatin is related to serum soluble transferrin receptors and with prohepcidin,Citation16 whereas in patients with gestational diabetes mellitus serum visfatin is associated with serum ferritin.Citation17 In the light of the above findings, the level of this multifactorial peptide in HD patients, and its associations with markers of iron metabolism, as well as with inflammation and situations characterized by insulin resistance were evaluated. Also the relation between visfatin levels and demands for recombinant human erythropoietin (rHuEpo) was assessed.
Patients and methods
Patients
Thirty-three HD patients (22 males) and 20 healthy volunteers (12 males) enrolled in the study. The two groups did not differ regarding age, which was 58.9 ± 13.4 years in HD patients and 55.3 ± 10.2 years in healthy volunteers (p = 0.267). Fifteen of the HD patients were anuric.
Patients underwent regular HD with polysulfone low-flux dialyzers (F low-flux series; Fresenius Medical Care, Bad Homburg, Germany) and with a bicarbonate dialysate containing 2.5 or 3 meq/L calcium for 4 h sessions, three times a week. Patients’ basic clinical characteristics and laboratory values expressed as mean ± SD are provided in .
Table 1. Patients’ basic clinical and laboratory characteristics.
From the 33 patients, 19 were receiving darbepoetin alfa (Aranesp; Amgen, Thousand Oaks, CA) (0.948 ± 0.705 mcg/kg/week), eight were receiving methoxy polyethylene glycol-epoetin beta (MIRCERA; F. Hoffmann-La Roche, Basel, Switzerland) (160.00 ± 94.83 U/month) and six were not receiving rHuEpo. In order to avoid the acute effect of intravenous iron on the evaluated factors iron treatment was withheld for a 4 weeks period prior to the study.
None of the patients suffered from active infection, malignancy, active autoimmune disease or was a hepatitis B, hepatitis C, or human immunodeficiency virus carrier. In order to exclude subclinical infection patients with white blood cell count higher than 10,000/mcL were excluded from the study. None of the patients was receiving corticosteroids or cytotoxic drugs for at least six months prior to the study and none of them received a blood transfusion for at least three months prior to the study. An informed consent was obtained from each individual enrolled in the study and the hospital ethics committee gave its approval to the study protocol.
Methods
Blood samples were drawn just before the onset of the second HD session of the week. Common hematological tests were performed immediately and harvested serum was stored at −80 °C.
Serum visfatin was assessed in HD patients and healthy volunteers by means of enzyme-linked immunosorbent assay (ELISA) (Phoenix Pharmaceuticals, Burlingame, CA). The sensitivity of the above ELISA kit is 1.85 ng/mL.
Serum bioactive 25 amino-acids hepcidin was measured in HD patients and healthy volunteers by means of ELISA (DRG Instruments GmbH, Marburg, Germany). The sensitivity of the above ELISA kit is 0.35 ng/mL. Serum IL-6 was also measured in HD patients and healthy volunteers with an ELISA kit (Bender Med Systems, Vienna, Austria). The sensitivity of the above ELISA kit is 0.92 pg/mL. Serum iron, ferritin, and TSAT were measured using an automatic analyzer (Roche Diagnostics, Mannheim, Germany). Hemoglobin was also assessed.
Recombinant human erythropoietin resistance index (ERI) was also calculated, albeit in 25 patients that were receiving darbepoetin alfa or no rHuEpo at all, since eight of the 33 patients were receiving methoxy polyethylene glycol-epoetin beta. ERI was calculated as the weekly dose of darbepoetin alfa per kg of dry body weight divided by hemoglobin.
Statistical analysis
One-sample Kolmogorov–Smirnov test confirmed the normality of the evaluated variables. For comparison of means unpaired t-test was used, whereas for evaluating relations linear regression analysis was performed. Results were expressed as mean ± SD and a p < 0.05 was considered significant.
Results
Visfatin levels in HD patients and healthy volunteers
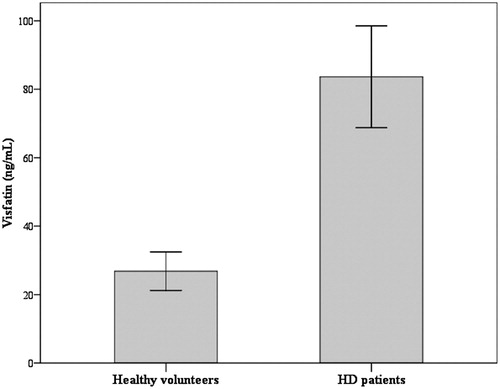
Compared to healthy volunteers, visfatin was markedly increased in HD patients. Serum visfatin was 77.91 ±30.04 ng/mL in HD patients and only 27.84 ± 11.67 ng/mL in healthy volunteers (p < 0.001, 95% confidence interval of difference from 39.42 ng/mL to 62.90 ng/mL) ().
Figure 1. Serum visfatin in HD patients and healthy volunteers. Notes: Serum visfatin was markedly increased in HD patients. It was 77.91 ± 30.04 ng/mL in HD patients and only 27.84 ± 11.67 ng/mL in healthy volunteers (p < 0.001, 95% confidence interval of difference from 39.42 ng/mL to 62.90 ng/mL).
Serum visfatin did not differ between HD patients with (n = 9) or without diabetes mellitus (80.67 ± 31.30 ng/mL vs. 76.88 ± 30.21 ng/mL respectively, p = 0.752). Similarly, serum visfatin did not differ between anuric (n = 15) and oliguric HD patients (79.87 ± 30.76 ng/mL vs. 76.28 ± 20.20 ng/mL respectively, p = 0.739). Finally, visfatin was higher in patients not receiving rHuEpo (n = 6) (112.5 ± 33.31 ng/mL vs. 73.14 ± 26.79 ng/mL, p = 0.012).
Relations of serum visfatin to the other evaluated variables in HD patients
In HD patients serum ferritin was significantly higher than in healthy volunteers (212.77 ± 220.85 ng/mL vs. 121.11 ±73.15 ng/mL, p = 0.034). Serum iron did not differ between HD patients and healthy volunteers (64.18 ± 51.11 ng/dL vs. 85.58 ± 32.90, p = 0.108). TSAT also did not differ between HD patients and healthy volunteers (20.92 ± 17.73% vs. 29.88 ± 11.11%, p = 0.053). Hepcidin was significantly higher in HD patients (273.28 ± 96.16 ng/mL vs. 104.37 ± 39.60 ng/mL, p < 0.001). Serum IL-6 was also significantly elevated in HD patients (50.75 ± 36.35 pg/mL vs. 14.27 ± 6.83 pg/mL, p < 0.001).
In HD patients, among the evaluated common markers of iron metabolism, serum visfatin was related only to TSAT (r = 0.366, p = 0.036). Visfatin was related neither to serum iron (r = 0.319, p = 0.070), nor to serum ferritin (r = 0.147, p = 0.416). In HD patients visfatin was not related to hepcidin (r = 0.138, p = 0.468). Serum visfatin was not related to BMI (r = 0.117, p = 0.515). In HD patients serum visfatin was not related to serum IL-6 as well (r = −0.122, p = 0.498).
Serum visfatin was strongly and positively related to hemoglobin (r = 0.643, p < 0.001). On the contrary, an inverse relation was detected between serum visfatin and the weekly dose of rHuEpo per kg of dry body weight (r = −0.452, p = 0.023). Consequently, visfatin was strongly and inversely related to ERI (r = −0.565, p = 0.003). Multivariate linear regression analysis revealed that visfatin was inversely related to ERI and independently of TSAT. Adjusted R2 was 0.447 (p = 0.001), standardized b was −0.374 (p = 0.049) for visfatin and −0.405 (p = 0.035) for TSAT. All the relations among the evaluated variables in HD patients are presented in .
Table 2. Relations among the evaluated variables in HD patients.
Discussion
In accordance with previous studies,Citation9–13 serum visfatin levels were markedly increased in HD patients. Visfatin levels did not differ between diabetics and non-diabetics HD patients and consequently the existence of diabetics in the HD patients group did not contribute to the elevated visfatin levels. This is in accordance with previous studies performed in diabetics and non-diabetics HD patientsCitation9,Citation10 but in discrepancy with the general population in which visfatin is increased in type II diabetic patients.Citation18 Visfatin is also increased in case of obesity and metabolic syndrome.Citation7,Citation19 However, we failed to detect a relation between visfatin and BMI in HD patients, which has also been detected by others.Citation9,Citation11 Thus it is likely that, although common in HD,Citation6 insulin resistance does not play a significant role in elevated visfatin levels among HD patients.
Another factor common in HD is inflammation.Citation7 Inflammation is accompanied by elevated visfatin levels.Citation1,Citation2 Visfatin per se up regulates the expression of various proinflammatory cytokines, the IL-6 included, in human monocytes.Citation20 Also, IL-6 is known to be markedly increased in HD patients.Citation21,Citation22 However, in this study no relation was detected between serum visfatin and IL-6 levels in HD patients. On the contrary, such a relation was detected by others,Citation8,Citation12 but not all investigators.Citation13 Thus the effect of inflammation on visfatin levels in HD patients remains to be elucidated.
Interestingly, visfatin levels did not differ between anuric and oliguric HD patients, indicating that at least in this population with end stage renal disease, the residual renal function does not affect visfatin levels. However, even in non-anuric HD patients the residual renal function is too small and consequently decreased elimination of visfatin by the kidneys could not be excluded as a cause of its increased level in HD patients. The exact reasons for elevated visfatin levels in HD patients remain to be elucidated.
In patients with insulin resistance visfatin was found to be related to markers of iron metabolism.Citation16,Citation17 Although a physiological explanation has not been provided yet, in the cohort of our patients visfatin was positively related to TSAT. Others did not detect such an association between visfatin and markers of iron metabolism in HD patients, although an almost statistically significant correlation was detected with prohepcidin.Citation12 However, in our study in order to avoid the acute effect of intravenous iron administration on the evaluated parameters, iron treatment was withheld for a 4 weeks period prior to the study.
In the cohort of our patients serum visfatin was strongly and positively related to hemoglobin. On the contrary, an inverse relation was detected between serum visfatin and the weekly dose of rHuEpo per kg of dry body weight. As a consequence, ERI was strongly and inversely related to visfatin. In order to determine if visfatin has a direct effect on erythropoiesis or if it acts indirectly by increasing iron availability, assessed by TSAT, multivariate regression analysis was performed and showed that in HD patients visfatin decreases the demands for rHuEpo independently of its effect on iron metabolism.
The opposite explanation that is the possibility exogenous rHuEpo to decrease visfatin levels could not be excluded and visfatin was higher in HD patients not receiving rHuEpo. However, data from the literature supports the possibility that visfatin decreases the demands for exogenous rHuEpo in HD patients by increasing erythropoiesis. As already noted, visfatin exerts insulin mimetic properties since it is capable of activating insulin receptor.Citation5,Citation23 Insulin receptors are present in red cells and their progenitorsCitation24 and insulin enhances cellular iron uptake by a mechanism that may involve the redistribution of transferrin receptors to the cell surface.Citation25 Such an action in progenitors of red cells would facilitate iron uptake and erythropoiesis. Insulin-like growth factor I (IGF-I) can also bind, albeit more weakly, insulin receptor and the structural similarity between insulin receptor and IGF-I receptor makes possible the binding of visfatin with both receptors.Citation26 Insulin-like growth factor I enhances erythropoiesis,Citation27,Citation28 and interestingly it plays a key role in regulating erythropoiesis in HD patients with erythrocytosis.Citation29 Additionally, stimulation of insulin receptor in erythrocytes augments glycolysis and prevents red cell hemolysis.Citation30 Although, the above data only indirectly suggest a role for visfatin in erythropoiesis and red cell life-span, the high visfatin mRNA expression in the bone marrow,Citation3 makes such a function possible. The intracellular role of visfatin in the NAD salvage pathway cannot be excluded as well.Citation4 This pathway plays significant role in red cellsCitation31 and inhibition of visfatin/Nampt decreases proliferation inducing among others anemia.Citation32
A limitation of our study is the relatively small number of the enrolled patients. However, the relations were statistically strong and further research on erythropoiesis in HD patients is required, due to the very frequent and costly complication of anemia. The possible serious side effects, related to higher risk of stroke, a higher rate of thromboembolism, and, possibly, a higher risk of cancer deaths in patients with a history of cancer, due to treatment with high doses of rHuEpo revealed by the TREAT study further indicate the significance of such a research.Citation33 Certainly, if our results will be confirmed by larger studies, experiments should be performed for revealing the exact mechanisms implicated in the effect of visfatin on erythropoiesis. In conclusion, visfatin is increased in HD patients and it is associated with decreased demands for rHuEpo.
Declaration of interest
The authors declare that they have no conflict of interest.
References
- Dahl TB, Holm S, Aukrust P, et al. Visfatin/NAMPT: a multifaceted molecule with diverse roles in physiology and pathophysiology. Annu Rev Nutr. 2012;32:229–243
- Sonoli SS, Shivprasad S, Prasad CV, et al. Visfatin – a review. Eur Rev Med Pharmacol Sci. 2011;15:9–14
- Samal B, Sun Y, Stearns G, et al. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–1437
- Revollo JR, Grimm AA, Imai S. The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Curr Opin Gastroenterol. 2007;23:164–170
- Fukuhara A, Matsuda M, Nishizawa M, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430
- Siew ED, Ikizler TA. Insulin resistance and protein energy metabolism in patients with advanced chronic kidney disease. Semin Dial. 2010;23:378–382
- Eleftheriadis T, Antoniadi G, Liakopoulos V, et al. Disturbances of acquired immunity in hemodialysis patients. Semin Dial. 2007;20:440–451
- Erten Y, Ebinc FA, Ebinc H, et al. The relationship of visfatin levels to inflammatory cytokines and left ventricular hypertrophy in hemodialysis and continuous ambulatory peritoneal dialysis patients. Ren Fail. 2008;30:617–623
- Kato A, Odamaki M, Ishida J, et al. Relationship between serum pre-B cell colony-enhancing factor/visfatin and atherosclerotic parameters in chronic hemodialysis patients. Am J Nephrol. 2009;29:31–35
- Niepolski L, Grzegorzewska AE, Mlot-Michalska M. Visfatin and endogenous secretory receptor for advanced glycation end-products in diabetic type 2 and non-diabetic patients undergoing intermittent hemodialysis. Int Urol Nephrol. 2010;42:441–452
- Carrero JJ, Witasp A, Stenvinkel P, et al. Visfatin is increased in chronic kidney disease patients with poor appetite and correlates negatively with fasting serum amino acids and triglyceride levels. Nephrol Dial Transplant. 2010;25:901–906
- Malyszko J, Malyszko JS, Mysliwiec M. Visfatin and endothelial function in dialyzed patients. Nephrology (Carlton). 2010;15:190–196
- Ziegelmeier M, Bachmann A, Seeger J, et al. Adipokines influencing metabolic and cardiovascular disease are differentially regulated in maintenance hemodialysis. Metabolism. 2008;57:1414–1421
- Tussing-Humphreys LM, Nemeth E, Fantuzzi G, et al. Elevated systemic hepcidin and iron depletion in obese premenopausal females. Obesity (Silver Spring). 2010;18:1449–1456
- Eleftheriadis T, Liakopoulos V, Antoniadi G, et al. The role of hepcidin in iron homeostasis and anemia in hemodialysis patients. Semin Dial. 2009;22:70–77
- Fernandez-Real JM, Moreno JM, Chico B, et al. Circulating visfatin is associated with parameters of iron metabolism in subjects with altered glucose tolerance. Diabetes Care. 2007;30:616–621
- Kaygusuz I, Gumus, II, Yilmaz S, et al. Serum levels of visfatin and possible interaction with iron parameters in gestational diabetes mellitus. Gynecol Obstet Invest. 2013;75:203--209
- Li L, Yang G, Li Q, et al. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Exp Clin Endocrinol Diabetes. 2006;114:544–548
- Li J, Ge X, Ma JM, et al. Rosai-Dorfman disease of unilateral lacrimal gland in an elderly Chinese male. Int J Ophthalmol. 2012;5:541–542
- Moschen AR, Kaser A, Enrich B, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748–1758
- Eleftheriadis T, Pissas G, Antoniadi G, et al. Damage-associated molecular patterns derived from mitochondria may contribute to the hemodialysis-associated inflammation. Int Urol Nephrol. 2013 . [Epub ahead of print]. doi: 10.1007/s11255-013-0417-z
- Eleftheriadis T, Kartsios C, Pissas G, et al. Increased plasma angiogenin level is associated and may contribute to decreased T-cell zeta-chain expression in hemodialysis patients. Ther Apher Dial. 2013;17:48–54
- Xie H, Tang SY, Luo XH, et al. Insulin-like effects of visfatin on human osteoblasts. Calcif Tissue Int. 2007;80:201–210
- Baumann G, MacCart JG. Kinetics of cell age-dependent decline of insulin receptors in human red cells. Am J Physiol. 1984;247:E667–E674
- Davis RJ, Corvera S, Czech MP. Insulin stimulates cellular iron uptake and causes the redistribution of intracellular transferrin receptors to the plasma membrane. J Biol Chem. 1986;261:8708–8711
- Ward CW, Lawrence MC. Ligand-induced activation of the insulin receptor: a multi-step process involving structural changes in both the ligand and the receptor. Bioessays. 2009;31:422–434
- Muta K, Krantz SB, Bondurant MC, et al. Distinct roles of erythropoietin, insulin-like growth factor I, and stem cell factor in the development of erythroid progenitor cells. J Clin Invest. 1994;94:34–43
- Kling PJ, Taing KM, Dvorak B, et al. Insulin-like growth factor-I stimulates erythropoiesis when administered enterally. Growth Factors. 2006;24:218–223
- Shih LY, Huang JY, Lee CT. Insulin-like growth factor I plays a role in regulating erythropoiesis in patients with end-stage renal disease and erythrocytosis. J Am Soc Nephrol. 1999;10:315–322
- Zancan P, Sola-Penna M. Regulation of human erythrocyte metabolism by insulin: cellular distribution of 6-phosphofructo-1-kinase and its implication for red blood cell function. Mol Genet Metab. 2005;86:401–411
- Micheli V, Simmonds HA, Sestini S, et al. Importance of nicotinamide as an NAD precursor in the human erythrocyte. Arch Biochem Biophys. 1990;283:40–45
- Holen K, Saltz LB, Hollywood E, et al. The pharmacokinetics, toxicities, and biologic effects of FK866, a nicotinamide adenine dinucleotide biosynthesis inhibitor. Invest New Drugs. 2008;26: 45–51
- Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032