Abstract
Arginine (ARG) and its methylated analogs (methylarginines) are the crucial regulators of nitric oxide (NO) bioavailability. ARG is the substrate for NO synthesis, whereas monomethylarginine (MMA) and asymmetric dimethylarginine (ADMA) are potent inhibitors. Symmetric dimethylarginine (SDMA) does not interfere with NO synthesis, but competes with ARG for the intracellular transport. The kidneys play the major role in ARG and methylarginines metabolism. They synthesize ARG de novo and eliminate methylarginines by excretion into urine and also by enzyme dimethylarginine dimethylaminohydrolase (DDAH) degrading only ADMA and MMA. Acute renal injury (ARI) is known to be accompanied by reduced NO production in the body. This study aimed to investigate the influence of ARI on ARG and methylarginines metabolism, and to establish the relationship between disturbances in the latter and reduced NO bioavailability in ARI. The rhabdomyolysis-related ARI model in rats was used. ARI reduced renal synthesis of ARG and its level in circulation as well as renal DDAH activity. However, ADMA did not accumulate because of its increased urinary excretion. Whole-body production of SDMA was increased significantly, whereas whole-body metabolism of MMA did not change. ARG and methylarginines content in renal tissue was decreased. Moreover, the balance between the substrate and inhibitors for NO synthesis was changed in favor of the inhibitors in renal tissue as well as in blood, and daily urinary excretion of NO metabolites was significantly decreased. Thus, ARI provokes severe disturbances in ARG and methylarginines metabolism that results in reduced NO bioavailability in the kidney and the whole body.
Introduction
The semi-essential amino acid l-arginine (ARG) is known to be the main source of nitric oxide (NO) in the body. This amino acid is the only substrate for NO-synthases (NOS), the family of intracellular NO-producing enzymes which convert ARG to citrulline with NO liberating.Citation1 The methylated analogs of ARG, also known as methylarginines, are the compounds which are similar to the ARG structure and therefore able to interfere with NO synthesis.Citation2 So far three methylarginines produced endogenously in mammals have been found: monomethylarginine (MMA), asymmetric dimethylarginine (ADMA), and symmetric dimethylarginine (SDMA). These compounds are formed throughout the body as a result of posttranslational modification of various proteins and liberated after proteolysis of these proteins.Citation3 MMA and ADMA exhibit both strong inhibitory activity on all NOS isoforms and affinity to transmembrane carriers of ARG that allows methylarginines to restrict ARG transport into the cell. SDMA is not a NOS inhibitor, but along with other methylarginines, SDMA competes with ARG for transmembrane carriers.Citation4 The described features of endogenous methylarginines suggest that these compounds are the important regulators of NO bioavailability in the body.
The major role in ARG and methylarginines metabolism plays the kidney. Proximal tubules of the kidney have been shown to be a predominant site of de novo ARG synthesisCitation5 and the most important source of circulating ARG.Citation6 In addition, ARG is almost completely reabsorbed from urine by the epithelium of proximal tubules and then returns into circulation. The renal tissue also contains large quantities of dimethylarginine dimethylaminohydrolase (DDAH), the only known enzyme degrading MMA and ADMA.Citation7 It has been shown that DDAH in some species eliminates more than 80% of endogenously produced ADMA.Citation8 The remaining ADMA and MMA are excreted in the urine. In contrast to ADMA, its structural isomer SDMA is not hydrolyzed by DDAH and eliminated exclusively by urinary excretion.Citation9
Since kidneys are essential for metabolism of ARG and methylarginines, the renal injury might affect levels of these compounds in circulation and different tissues. There are many studies in line with this concept, which revealed accumulation of SDMA and ADMA in the blood of animals and people with chronic kidney disease.Citation10 The high circulating concentration of methylarginines resulted in decreased NO synthesis and was associated with cardiovascular complications, such as endothelial dysfunction, atherosclerosis, hypertension, and others.Citation11 Beside these findings, Brunini et al.Citation12 observed decreased circulating level of ARG in hemodialysis patients that could also reduce NO production and bioavailability.
Acute renal injury (ARI) has also been shown to be accompanied by decreased NO production in the body,Citation13 but there are no studies undertaken so far to elucidate whether ARG and its methylated analogs are involved in decreased whole-body NO synthesis in ARI. The present study was designed to investigate the influence of ARI on ARG and methylarginines metabolism in rats, and to establish the relationship between disturbances in the metabolism of these compounds and reduced NO bioavailability in ARI.
Materials and methods
Animals and experimental protocol
Male Wistar rats (180–220 g body weight) from the Animal House of the Institute of Physiology (Novosibirsk, Russia) were housed individually in metabolic cages (Tecniplast, Buguggiate, Italy) with free access to water and laboratory chow (ProKorm granules, BioPro, Novosibirsk, Russia). The experimental protocol was approved by the ethical committee of the Institute of Physiology and carried out in accordance with the European Community Council Directive 86/609/EEC.
ARI (n = 11) was induced through intramuscular injection of 50% (v/v in sterile saline) glycerol in both hind limbs (totally 10 mL/kg body weight). Such an injection induces abundant rhabdomyolysis, the liberation of myoglobin into circulation and results in acute tubular necrosis.Citation14 All animals were deprived of drinking water for 24 h before the injection. Animals were allowed free access to water immediately after glycerol injection. Control animals (n = 8) received saline instead of glycerol in the same volume.
At 72 h after the injection, the daily urine output was evaluated and urine samples were collected. Then the animals were sacrificed under anesthesia to collect blood and renal tissue samples. Blood samples were collected in tubes containing 40 µL of 5% ethylenediaminetetraacetic acid and centrifuged immediately at 1000 × g for 20 min at 4 °C. Obtained plasma and urine samples were frozen at −20 °C until analysis. The right kidney was extracted through translumbar incision and rinsed with saline. The top third of the kidney was homogenized on ice with four volumes of 0.1 M of Na2HPO4 buffer (pH 6.5) containing 0.5 mM of 1,4-dithiothreitol. The homogenates were centrifuged at 9100 × g for 40 min at 4 °C. Supernatant was stored at −70 °C until analysis.
Renal function assessment
Plasma and daily urine were assayed for creatinine (CR) and urea by using standard diagnostic kits (Fluitest, Biocon, Germany). Renal clearance of compounds (CLX, mL/min) was calculated using the following formula: CLX = [UX] V/[PX]/1440, where [UX] is the concentration of X in the urine; [PX] is the concentration of X in the plasma; V is the urine flow rate (mL/min). Glomerular filtration rate (GFR) was estimated using endogenous CR clearance. Fractional excretion of compounds (FEX, %) was calculated using the following formula: FEX = 100CLX/GFR. Reabsorption of glomerular filtrate (R, %) was calculated using the following formula: R = 100 (GFR − V)/GFR.
ARG, MMA, ADMA, and SDMA determination
Concentrations of ARG and its methylated analogs in plasma, urine, and renal tissue were measured by high-performance liquid chromatography (HPLC) with fluorescence detection after solid-phase extraction (SPE) according to Teerlink.Citation15 Plasma and urine samples were centrifuged briefly before analysis for removing any debris. Tissue homogenates were deproteinized by adding 10 µL of 30% 5-sulfosalicilic acid (SSA) to 300 µL of homogenate. Then homogenates were stirred and centrifuged at 9100 × g for 10 min at 4 °C. 200 µL of the sample was mixed with 100 µL of 40-µM solution of the internal standard (homoarginine) and with either 700 µL of phosphate-buffered saline (pH 7.0) for plasma and urine samples or 700 µL of 0.1 M Na2HPO4 for tissue samples. This mixture was applied to Oasis MCX solid-phase extraction columns (1 cc, 30 mg; Waters, Milford, MA) conditioned with 1 mL methanol and 1 mL of water. The columns were consecutively washed with 1 mL of 100 mM HCl and 1 mL of methanol. Analytes were eluted with 1 mL of concentrated ammonia/water/methanol/1 M NaOH (10/40/50/0.5). After evaporation of the solvent under nitrogen, the amino acids were redissolved in 100 µL of water and derivatized with ortho-phthaldialdehyde reagent containing 3-mercaptopropionic acid. The derivatives were separated using isocratic reversed-phase chromatography on Luna C18(2) column (3-µm particle size, 100 × 2 mm; Phenomenex, Torrance, CA), injection volume of 20 µL (auto-sampler SIL-10AD, Shimadzu, Kyoto, Japan). 50 mM KH2PO4 buffer (pH 6.4) containing 8.7% acetonitrile was used as the mobile phase at a flow rate of 0.2 mL/min (LC-10ADvp pump, Shimadzu, Kyoto, Japan) and a column temperature of 45 °C. Fluorescence detection was performed at excitation and emission wavelengths of 340 and 455 nm, respectively (RF-10 A detector, Shimadzu, Kyoto, Japan). After elution of the last analyte, the column was washed with 25% acetonitrile in KH2PO4 buffer. The concentration of the analytes was calculated using peak area of standards (100 µM of ARG, 10 µM of each MMA, ADMA, and SDMA) and unknown samples equalized with internal standard.
Renal tissue DDAH activity assay
DDAH activity in renal tissue was determined by the direct measurement of enzymatically degraded ADMA as described by Nonaka et al.Citation16 The samples were thawed in an ice bath before analysis. Each sample was aliquoted twice: 90 µL of the rat kidney homogenate and 180 µL of 0.1 M Na2HPO4 buffer (pH 6.5) were added to 30 µL of 500 µM ADMA. To inactivate DDAH, 30 µL of 30% SSA was immediately added to the first aliquot, which was then incubated at 37 °C for 60 min. This sample provided a baseline of 0% DDAH activity. The second aliquot was incubated at 37 °C for 60 min before the addition of 30% SSA. All samples were centrifuged at 9100 × g for 10 min at 4 °C, and supernatant was subjected to SPE procedure and HPLC analysis described above. DDAH activity was expressed as a decrease in ADMA quantum (pmol) per minute per mg protein.
Renal ARG production assay
The rate of ARG production in renal tissue was determined by a technique similar to DDAH activity assay described above, but without any addition of exogenous ADMA. The rate of ARG production was expressed as an increase in ARG quantum (pmol) per minute per mg protein.
Protein content determination
Concentrations of protein in tissue samples were determined by BradfordCitation17 using crystalline BSA as a standard.
Total nitrite/nitrate (NOx) assay
NOx levels were measured in plasma and daily urine. All samples were deproteinized by adding 15 µL of 2 M ZnSO4 to 300 µL of the sample (urine samples were diluted 10-fold with deionized water before addition). Samples were stirred for 2 min, incubated for 10 min at room temperature, and centrifuged at 12,000 × g for 14 min. 250 µL of supernatant was mixed with 84 µL of 0.2 M glycine–NaOH buffer (pH 9.7).
Nitrate was reduced to nitrite by copper-coated cadmium.Citation18 For activation, cadmium granules (d ∼ 2 mm) were rinsed with deionized water and swirled in 5 mM CuSO4 (dissolved in glycine–NaOH buffer) for 5 min. Then copper-coated granules were rinsed with 0.2 M glycine–NaOH buffer, dried on air, and immediately used. Freshly activated granules (4 pcs) were placed in a tube with the sample and stirred for 15 min. Then the sample was transferred into a new tube and centrifuged at 12,000 × g for 7 min in order to precipitate the metallic dust. Supernatant was stored for nitrite determination. Used granules were rinsed with deionized water and stored in 0.1 M H2SO4. They can be regenerated by repeating the Cu-depositing step described above.
Nitrite was measured using the Griess reaction.Citation18 150 µL of supernatant (after reduction by cadmium) was applied to a microtiter plate well followed by 75 µL of 60 mM sulfanilamide (in 7% HCl) and 75 µL of 0.77 mM N-naphthyl ethylenediamine dihydrochloride (NEDA). After 5 min of color development at room temperature, the absorbance was measured on a microplate reader (ELx 808iu, Bio-Tek, Winooski, VT) using a test wavelength of 540 nm and a reference wavelength of 630 nm. Calibration curves were made over a linear range of nitrate (KNO3 solution) between 0 and 100 µM.
Statistical analysis
Statistical analysis was performed using Statistica 10 (StatSoft, Tulsa, OK). Normality of the data was assessed using the Kolmogorov–Smirnov (K–S) test. Differences between the groups were tested using t-test for independent samples. Relationships between parameters were assessed using Pearson’s correlation coefficient (r) among all sampling. Values are expressed as means ± SE, and a difference was considered significant at p < 0.05 level.
Results
Renal function after glycerol administration
Intramuscular injection of glycerol produced a marked derangement in renal excretory function. At 72 h after the injection, GFR and renal clearance of urea in animals were significantly reduced that leaded to decreased daily urinary excretion of CR and urea, and accumulation of these compounds in plasma (). Urine output did not differ significantly between groups, but estimated reabsorption of glomerular filtrate in kidneys of ARI animals was decreased.
Table 1. Effect of ARI on renal excretory function.
Influence of ARI on renal excretion and circulating levels of ARG and methylarginines
Despite marked derangement in renal excretory function in ARI animals, daily urinary excretion of ARG and methylarginines did not decrease (). Moreover, the amount of ADMA excreted in the urine was negligible in control animals and increased more than 100 times in ARI animals.
Figure 1. Effect of ARI on daily urinary excretion of arginine and methylarginines. Data are expressed as mean ± SE. ARG = arginine, MMA = monomethylarginine, ADMA = asymmetric dimethylarginine, SDMA = symmetric dimethylarginine. ***p < 0.001, t-test for independent samples.
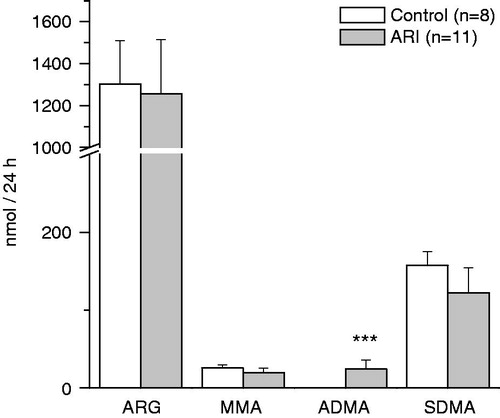
The concentrations of amino acids in daily urine were adjusted to CR. The adjusted concentrations of ARG and its methylated analogs in urine of ARI rats were significantly increased (). Fractional excretion of all these compounds was also increased and had the strong negative linear relationships with the reabsorption of glomerular filtrate: r = −0.94 for ARG, r = −0.93 for MMA, r = −0.90 for ADMA, and r = −0.82 for SDMA, p < 0.05 for each value. The renal clearance of ADMA was significantly higher in ARI as compared to control, whereas the clearance of SDMA was significantly lower (). The clearance of ARG and MMA did not change significantly in ARI.
Table 2. Effect of ARI on concentration in urine, fractional excretion, and renal clearance of arginine and methylarginines.
The concentration of ARG in the plasma of ARI animals was decreased (). On the contrary, the circulating level of SDMA was significantly increased. The plasma concentration of MMA as well as ADMA did not change significantly in ARI as compared to control. As a result of described changes in circulating level of ARG, the ratio of NOS substrate to inhibitors (MMA + ADMA) was decreased in plasma of ARI animals (). In addition, ARG/methylarginines ratio was also decreased, whereas SDMA/ADMA ratio and SDMA/MMA ratio were increased. The correlation analysis revealed the significant relationships between the ARG/methylarginines ratio and the markers of renal function: r = −0.81 for CR and r = −0.82 for urea in plasma, p < 0.05 for both. The concentration of SDMA in circulation was also related to these markers: r = 0.96 for CR and r = 0.95 for urea, r = −0.90 for GFR, p < 0.05.
Figure 2. Effect of ARI on circulating level of arginine and methylarginines. Data are expressed as mean ± SE. ARG = arginine, MMA = monomethylarginine, ADMA = asymmetric dimethylarginine, SDMA = symmetric dimethylarginine. **p < 0.01, ***p < 0.001, t-test for independent samples.
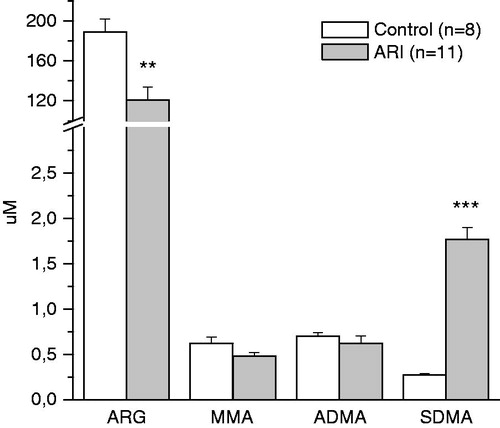
Table 3. Ratios of arginine and methylarginines in blood and renal tissue of control rats and rats with ARI.
Influence of ARI on ARG and methylarginines content in renal tissue
The concentration of ARG in renal tissue of both groups of animals was much higher than that of methylarginines (). However, ARI caused the significant decrease in the content of all these compounds: ARG was lowered by 66.4 ± 2.4%, MMA by 40.6 ± 6.4%, ADMA by 58.0 ± 7.9%, and SDMA by 59.6 ± 1.1%, p < 0.00001. The decrease in content of ARG and methylarginines in renal tissue of ARI animals was accompanied by alterations in ratios of these compounds (). The ratio of NOS substrate to inhibitors was significantly reduced as well as ARG/methylarginines ratio.
Figure 3. Effect of ARI on renal tissue content of arginine and methylarginines. Data are expressed as mean ± SE. ARG = arginine, MMA = monomethylarginine, ADMA = asymmetric dimethylarginine, SDMA = symmetric dimethylarginine. ***p < 0.001, t-test for independent samples.
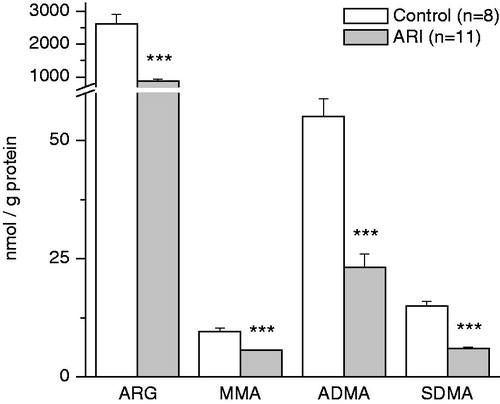
To further estimate the changes in the production of methylarginines in renal tissue, the ARG methylation index (ARG MI) was calculated using the concentrations of ARG and methylarginines in tissue samples. ARG MI is an integrative estimation of products derived from ARG by methylation expressed as a ratio of dimethylated (ADMA and SDMA) modifications of ARG to their monomethylated precursor (MMA), i.e., ARG MI = (ADMA + SDMA)/MMA. This index was significantly decreased in renal tissue of ARI animals as compared to control. Moreover, the amount of each dimethylarginine per unit of its precursor (MMA) was also decreased, whereas SDMA/ADMA ratio did not change ().
Influence of ARI on DDAH activity and the rate of ARG production in renal tissue
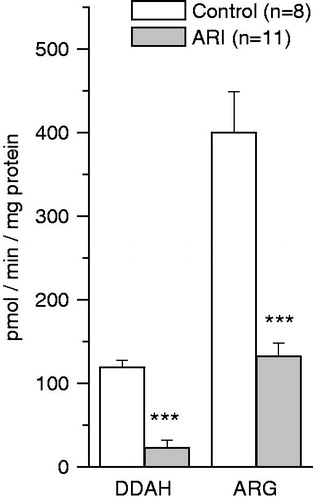
DDAH in renal tissue of control animals metabolized 119.2 ± 8.4 pmol ADMA/min/mg protein (). The same tissue also produced 399.6 ± 49.6 pmol ARG/min/mg protein (). However, in ARI animals, renal DDAH activity as well as the rate of ARG production was significantly reduced.
Figure 4. Effect of ARI on dimethylarginine dimethylaminohydrolase (DDAH) activity and the rate of arginine (ARG) synthesis in renal tissue. Data are expressed as mean ± SE. ***p < 0.001, t-test for independent samples.
The correlation analysis revealed the strong negative relationship between DDAH activity in renal tissue and the renal clearance of ADMA: r = −0.72, p < 0.05. There were also correlations between the DDAH activity and the daily urinary excretion of ADMA (r = −0.67, p < 0.05), and between the DDAH activity and the fraction excretion of ADMA (r = 0.72, p < 0.05). The correlation analysis did not reveal any significant relationships between the renal DDAH activity in the kidneys and the renal excretion of MMA, another substrate for DDAH. In addition, the circulating level of neither MMA nor ADMA was related to renal DDAH activity. The rate of ARG production in renal tissue also did not have the significant relationship with the level of ARG in circulation.
Influence of ARI on renal excretion and circulating level of NOx
The adjusted concentration of NOx and the amount of these NO metabolites in daily urine were decreased significantly in ARI rats, although their fractional excretion rose many times (). The plasma level of NOx did not differ significantly between the groups of animals: 23.69 ± 10.59 µM in control versus 12.60 ± 6.54 µM in ARI, p = 0.377. The correlation analysis revealed significant relationships between the daily excretion of NOx and both the renal DDAH activity (r = 0.90, p < 0.05) and the rate of ARG production in renal tissue (r = 0.85, p < 0.05). The NOx excretion was also related to GFR: r = 0.85, p < 0.05.
Table 4. Effect of ARI on renal excretion of total nitrite/nitrate (NOx).
Discussion
The glycerol-induced model of ARI used in the study is characterized by the critical reduction in GFR and the marked accumulation of toxic compounds, such as urea. However, the renal excretion of ARG and methylarginines was not decreased in ARI in spite of low GFR. Our data suggest that the deficiency in tubular amino acid reabsorption caused by tubular injury in ARI compensates low GFR and allows ARG and its analogs to excrete with urine. Indeed, the FEs of these amino acids in control animals was much less than 100% and was increased significantly in ARI, whereas the estimated reabsorption of glomerular filtrate was decreased.
In order to evaluate the whole-body metabolism of the compounds of interest, we have compared their daily amount excreted in urine with their concentration in blood. The data obtained clearly demonstrate that ARI affects the metabolism of ARG, since its concentration in blood was significantly reduced whereas its amount excreted in urine did not differ from that in control animals. The low level of circulating ARG was also found in ARI induced by uranyl nitrateCitation19 as well as in patients with chronic kidney disease.Citation12
One of the pathological mechanisms involved in the decreasing of ARG in circulation is the reduced ARG production by the kidney. In our study, the rate of ARG production by renal tissue was reduced more than three times in ARI rats as compared to control. These data are consistent with the study demonstrating that the kidney switches from ARG production into its uptake after ischemia--reperfusion.Citation20 We suppose that the low rate of ARG production by the kidney in ARI could be caused by a number of factors, such as the damage of tubular cells, the reduced activity of argininosuccinate synthase and argininosuccinate lyase, and the deficiency in the reabsorption of citrulline.
The lack of the linear relationship between renal ARG production and its plasma concentration suggests the other factors also influencing on the ARG level in circulation. The intensive uptake of ARG by hepatocytes has been demonstrated in rats with acute uremiaCitation21 as well as both the activation of ARG transport via system y(+) into red blood cells and low circulating ARG level in patients with chronic kidney disease.Citation12 Reduced dietary ARG intake due to the malnutrition, which is always associated with uremia,Citation22 also might contribute to the low ARG concentration in the blood of ARI animals.
The whole-body metabolism of dimethylated analogs of ARG was also affected by ARI. The salient finding of this study is the observation that the circulating level of ADMA was not changed significantly in ARI, but its amount excreted in urine rose from the negligible value to 24.42 ± 11.77 nmol per 24 h. We suggest that the increase in the urinary excretion of ADMA in ARI is caused by reduced renal DDAH activity. Indeed, we observed the 5-fold decrease in renal DDAH activity in ARI. Moreover, the correlation analysis revealed the strong negative relationship between the renal clearance of ADMA and renal DDAH activity.
DDAH has been shown to be expressed widely in the kidneyCitation23 and very sensitive to oxidative stress.Citation24 The reduction in the activity of this enzyme could be caused by intrarenal inflammation, oxidative stress, and the destruction of tubular epithelium, which are associated with glycerol-induced ARI.Citation25 The other mechanism suppressing the hydrolysis of ADMA by DDAH could be the activation of renin–angiotensin system associated with renal injury,Citation25 since angiotensin II was shown to decrease the expression of DDAH in renal tissue.Citation26 The decreased expression of DDAH and increased renal excretion of ADMA were also found in rats subjected to subtotal nephrectomy.Citation27 However, the level of ADMA in circulation was found to be elevated in that study.
It is noteworthy that reduced renal DDAH activity in ARI rats was not associated with the significant accumulation of ADMA in circulation, because DDAH is considered to be the main regulator of circulating ADMA level.Citation7,Citation11 We suggest that decreased tubular reabsorption of ADMA in ARI kidneys could prevent the accumulation of ADMA in circulation. We further suggest that diminished DDAH activity in the kidney might also be compensated by the increased expression and/or activity of this enzyme in other organs, such as liver. Both the increase in the utilization of ADMA from the circulation and the decrease in its concentration in blood were found in endotoxemia in rats, while GFR and the utilization of ADMA by the kidneys were significantly decreased.Citation28
The other intriguing finding of our study is the significant increase in whole-body production of SDMA in ARI. SDMA is considered to be an endogenous marker of renal function, since its elimination from the body is provided exclusively by renal excretionCitation9 and its level in circulation has a strong linear correlation with GFR and plasma CR.Citation29 The accumulation of SDMA in the blood of patients with chronic renal failure has been demonstrated in a number of clinical trials (reviewed by Schwedhelm & BögerCitation10). Some authors registered the changes in circulating SDMA level shortly after the decrease in GFR.Citation30,Citation31
We also observed the strong correlations between circulating SDMA and circulating levels of CR and urea as well as between SDMA and GFR. However, the significantly increased level of SDMA in circulation, in our study, could not be the result of impaired renal excretion, because the amount of SDMA excreted per 24 h did not change in ARI. The increase in the whole-body production of SDMA in ARI could be caused by the intensive proteolysis of SDMA-containing proteins, since the metabolic acidosis associated with uremia intensifies the breakdown of proteins in muscles.Citation32 Beside this, the malnutrition in uremia could also result in the intensive protein breakdown.Citation22 The destruction of hind limb muscles by glycerol (rhabdomyolysis) in the ARI model used in our study was probably accompanied by the extensive breakdown of muscle proteins. However, the rhabdomyolysis is unlikely to be the main casual factor for the elevation of SDMA in circulation, since both the increased SDMA concentration in blood and its unchanged renal excretion were found in rats subjected to subtotal nephrectomy, i.e., in the model where the extensive damage of muscles was excluded.Citation33 In the same model the increased gene expression of protein methyltransferases, the enzymes methylating ARG-residues in proteins, was also revealed in the liver and the kidneyCitation27 that proves these organs to be involved in the intensive production of methylarginines in uremia.
We measured the content of free ARG and methylarginines in renal tissue to elucidate whether the damaged kidney is one of the sources of methylarginines in ARI. The content of all these compounds was decreased by about 50%. The low concentration of ARG in renal tissue has been shown earlierCitation19 in uranyl nitrate-induced ARI, whereas our data, as far as we know, are the first demonstration of free levels of methylarginines in ARI kidneys. The deficiency in ARG could be a causing factor for the flux of this compound in the kidney after ischemia.Citation20 We suppose that decrease in the renal content of free ARG and methylarginines in ARI is a result of the impaired tubular reabsorption of these compounds and/or the reduced ARG synthesis by renal tissue. We also speculate that free methylarginines might be liberated from tissue into circulation after renal injury. This assumption is confirmed by the fact that the kidneys capture ADMA and SDMA from circulation under physiological conditions, but release them after ischemia.Citation34 In addition, our data demonstrate that ARI enhances the protein methylation in renal tissue, because the ARG/methylarginines ratio in renal tissue was decreased as well as ARG MI. Observed changes in the latter also reflect the more intensive monomethylation of ARG in ARI than its dimethylation.
It is noteworthy that neither increased monomethylation of renal proteins nor decreased renal DDAH activity in ARI influenced on MMA level in circulation and its amount excreted in urine. Moreover, since MMA is the only precursor of ADMA and SDMA,Citation3 the changes in their metabolism probably might have affected the whole-body metabolism of MMA, but it was not observed. Perhaps, the intensity of ARG monomethylation and dimethylation in proteins is regulated independently from each other, however, the data concerning this issue have not been presented so far. Our data also suggest that DDAH in kidneys does not play the major role in MMA elimination in rats. The correlation analysis did not reveal any significant relationship between renal MMA clearance and renal DDAH activity, whereas the latter was related to ADMA clearance.
In the present study we, along with others,Citation13 utilized the daily renal excretion of stable NO metabolites to estimate the whole-body NO synthesis, and observed its reduction in ARI. The above-described changes in the metabolism of ARG and its methylated analogs in ARI lead to the decrease in ARG/(ADMA + MMA) ratio in circulation as well as in renal tissue. This ratio between the NOS substrate and the NOS inhibitors is known to reflect the NO bioavailability in the body. Richir et al.Citation35 demonstrated that the decreasing of ARG/ADMA ratio in circulation was associated with the reducing of blood flow through the kidney, the liver, and the spleen. Thus, the change in balance between the substrate and inhibitors of NOS in favor of the latter could, along with other factors, cause the preglomerular vasoconstriction, endothelial dysfunction, and decreased NO production by glomeruli, which are typical of rhabdomyolysis-related kidney injury.Citation36,Citation37 Exogenous ARG, probably, is able to compensate the ARG deficiency in ARI, since the positive effects of ARG administration mediated by enhanced NO production were demonstrated repeatedly.Citation13,Citation37 The reducing of oxidative stress had also favorable effect on ARI.Citation38 Our data imply that one causing factor determining such an effect might be the restoring of renal DDAH activity, since the latter had a significant correlation with the daily excretion of NOx. However, this hypothesis still needs to be tested further.
In conclusion, our study discovered that rhabdomyolysis-related ARI provokes severe disturbances in the metabolism of ARG and methylarginines, which lead to reduced NO bioavailability in the injured kidney as well as in the whole body.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–837
- Caplin B, Leiper J. Endogenous nitric oxide synthase inhibitors in the biology of disease: markers, mediators, and regulators? Arterioscler Thromb Vasc Biol. 2012;32(6):1343–1353
- Nicholson TB, Chen T, Richard S. The physiological and pathophysiological role of PRMT1-mediated protein arginine methylation. Pharmacol Res. 2009;60(6):466–474
- Closs EI, Basha FZ, Habermeier A, Förstermann U. Interference of l-arginine analogues with l-arginine transport mediated by the y+ carrier hCAT-2B. Nitric Oxide. 1997;1(1):65–73
- Dhanakoti SN, Brosnan JT, Herzberg GR, Brosnan ME. Renal arginine synthesis: studies in vitro and in vivo. Am J Physiol. 1990;259(3 Pt 1):E437–E442
- Wu G, Morris Jr SM. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(Pt 1):1–17
- Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol. 2007;293(6):H3227–H3245
- Achan V, Broadhead M, Malaki M, et al. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol. 2003;23(8):1455–1459
- Yudkoff M, Nissim I, Pereira G, Segal S. Urinary excretion of dimethylarginines in premature infants. Biochem Med. 1984;32(2):242–251
- Schwedhelm E, Böger RH. The role of asymmetric and symmetric dimethylarginines in renal disease. Nat Rev Nephrol. 2011;7(5):275–285
- Leiper J, Nandi M. The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nat Rev Drug Discov. 2011;10(4):277–291
- Brunini TM, Roberts NB, Yaqoob MM, Ellory JC, Mann GE, Mendes Ribeiro AC. Activation of l-arginine transport in undialysed chronic renal failure and continuous ambulatory peritoneal dialysis patients. Clin Exp Pharmacol Physiol. 2006;33(1–2):114–118
- Chander V, Chopra K. Molsidomine, a nitric oxide donor and l-arginine protects against rhabdomyolysis-induced myoglobinuric acute renal failure. Biochim Biophys Acta. 2005;1723(1–3):208–214
- Dai C, Kiss LP, Liu Y. Animal models of kidney diseases. In: Conn PM, ed. Sourcebook of Models for Biomedical Research. New Jersey: Humana Press; 2008:657–665
- Teerlink T. HPLC analysis of ADMA and other methylated l-arginine analogs in biological fluids. J Chromatogr B. 2007;851(1–2):21–29
- Nonaka S, Tsunoda M, Aoyama C, Funatsu T. Determination of NG,NG-dimethyl-l-arginine in rat plasma and dimethylarginine dimethylaminohydrolase activity in rat kidney using a monolithic silica column. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;843(2):170–174
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254
- Navarro-Gonzálvez JA, García-Benayas C, Arenas J. Semiautomated measurement of nitrate in biological fluids. Clin Chem. 1998;44(3):679–681
- Schramm L, La M, Heidbreder E, et al. l-arginine deficiency and supplementation in experimental acute renal failure and in human kidney transplantation. Kidney Int. 2002;61(4):1423–1432
- Prins HA, Nijveldt RJ, Gasselt DV, et al. The flux of arginine after ischemia--reperfusion in the rat kidney. Kidney Int. 2002;62(1):86–93
- Perez GO, Rietberg B, Owens B, Schiff ER. Effect of acute uremia on arginine metabolism and urea and guanidino acid production by perfused rat liver. Pflugers Arch. 1977;372(3):275–278
- Elliott DA. Nutritional considerations for the dialytic patient. Vet Clin North Am Small Anim Pract. 2011;41(1):239–250
- Leiper JM, Santa Maria J, Chubb A, et al. Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem J. 1999;343(Pt 1):209–214
- Sydow K, Münzel T. ADMA and oxidative stress. Atheroscler Suppl. 2003;4(4):41–51
- Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361(1):62–72
- Onozato ML, Tojo A, Leiper J, Fujita T, Palm F, Wilcox CS. Expression of NG,NG-dimethylarginine dimethylaminohydrolase and protein arginine N-methyltransferase isoforms in diabetic rat kidney: effects of angiotensin II receptor blockers. Diabetes. 2008;57(1):172–180
- Matsuguma K, Ueda S, Yamagishi S, et al. Molecular mechanism for elevation of asymmetric dimethylarginine and its role for hypertension in chronic kidney disease. J Am Soc Nephrol. 2006;17(8):2176–2183
- Siroen MP, Teerlink T, Nijveldt RJ, Prins HA, Richir MC, van Leeuwen PA. The clinical significance of asymmetric dimethylarginine. Annu Rev Nutr. 2006;26:203–228
- Kielstein JT, Salpeter SR, Bode-Boeger SM, Cooke JP, Fliser D. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function – a meta-analysis. Nephrol Dial Transplant. 2006;21(9):2446–2451
- Carello KA, Whitesall SE, Lloyd MC, Billecke SS, D'Alecy LG. Asymmetrical dimethylarginine plasma clearance persists after acute total nephrectomy in rats. Am J Physiol Heart Circ Physiol. 2006;290(1):209–216
- Kielstein JT, Veldink H, Martens-Lobenhoffer J, et al. SDMA is an early marker of change in GFR after living-related kidney donation. Nephrol Dial Transplant. 2011;26(1):324–328
- Mitch WE, May RC, Maroni BJ, Druml W. Protein and amino acid metabolism in uremia: influence of metabolic acidosis. Kidney Int Suppl. 1989;27:S205–S207
- Al Banchaabouchi M, Marescau B, Possemiers I, D'Hooge R, Levillain O, De Deyn PP. NG, NG-dimethylarginine and NG, NG-dimethylarginine in renal insufficiency. Pflugers Arch. 2000;439(5):524–531
- Yeung KK, Richir M, Hanrath P, et al. Infrarenal aortic-clamping after renal ischemia aggravates acute renal failure. Eur J Clin Invest. 2011;41(6):605–615
- Richir MC, van Lambalgen AA, Teerlink T, et al. Low arginine/asymmetric dimethylarginine ratio deteriorates systemic hemodynamics and organ blood flow in a rat model. Crit Care Med. 2009;37(6):2010–2017
- Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14(8):2199–2210
- Valdivielso JM, Lopez-Novoa JM, Eleno N, Perez Barriocanal F. Role of glomerular nitric oxide in glycerol-induced acute renal failure. Can J Physiol Pharmacol. 2000;78(6):476–482
- Chander V, Chopra K. Protective effect of resveratrol, a polyphenolic phytoalexin on glycerol-induced acute renal failure in rat kidney. Ren Fail. 2006;28(2):161–169