Abstract
Occasionally, patients with acute Epstein–Barr virus (EBV) infection develop hemophagocytic syndrome (HPS). Acute kidney injury (AKI) is considered a strong prognostic factor, but very few data are available about the biopsy-proven renal involvement of EBV-HPS. Here we describe a previously healthy 17-year-old girl with EBV-HPS. Combination therapy failed and renal necropsy was performed. The renal histology showed that intact glomeruli, remarkable interstitial edema and some cellular infiltration, and protein casts. These findings were compatible with cytokine nephropathy as recently advocated. We suggest that hypercytokinemia may play an important role in the pathophysiology in AKI of EBV-HPS.
Introduction
Hemophagocytic syndrome (HPS) is a clinico-pathological entity caused by excessive activation and proliferation of non-malignant macrophage – phagocytosing blood cells. Activated T-lymphocytes and macrophages produce a huge amount of serum pro-inflammatory cytokines.Citation1 Most cases of HPS are associated with underlying disorders.Citation2 Epstein–Barr virus (EBV)-associated HPS is a rare disorder but it often takes an aggressive clinical course. Acute kidney injury (AKI) is considered a strong prognostic factor,Citation3 but very few data are available about the biopsy-proven renal involvement of EBV-HPS. Here we report a case of fatal EBV-HPS complicated by persisting renal dysfunction. We discuss the renal necropsy findings.
Case report
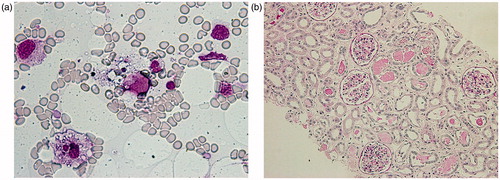
A previously healthy 17-year-old woman was admitted to our hospital with a high fever of 39 °C, vomiting, diarrhea, and joint pain for five days. Two days before admission, a general practitioner diagnosed her illness as infectious mononucleosis. There was neither family history of renal diseases nor immunological disorders. On admission, blood pressure was 86/40 mmHg, pulse rate 88/min, body temperature 37.4 °C, height 163 cm and weight 48.5 kg. She was alert. Examination of her heart and lungs was unremarkable. She had neck lymphadenopathy and splenomegaly. Laboratory findings were as follows: hemoglobin 11.5 g/dL (115 g/L), white blood cell count 2.0 × 103/μl (2.0 × 109/L), with 93.6% neutrophils, 4.9% lymphocytes, 1.5% monocytes, and platelet count 22 × 103/μl (22 × 109/L). The prothrombin time was 82.4% and the partial thromboplastin time was 30 s. Fibrinogen concentration was 53 mg/dL (1.56 µmol/L). Urine analysis showed a pH of 5.0, specific gravity of 1.027, protein 1+ and blood negative by dipstick with a sediment of white blood cells (28/high power field) and a few granular casts (2/high power field). Total bilirubin was 1.0 mg/dL (17.1 µmol/L), aspartate aminotransferase 147 IU/L, lactate dehydrogenase 1756 IU/L, blood urea nitrogen 65 mg/dL (23.2 mmol/L), creatinine 3.1 mg/dL (274 µmol/L), uric acid 14 mg/dL (833 µmol/L), creatine kinase 100 U/L, sodium 129 mEq/L (129 mmol/L), potassium 4.1 mEq/L (4.1 mmol/L), glucose 111 mg/dL (6.16 mmol/L), triglyceride 294 mg/dL (3.32 mmol/L), C-reactive protein 8.8 mg/dL, ferritin 11,000 ng/mL (11,000 µg/L). The anti-neutrophil cytoplasmic antibody, the anti-glomerular basement membrane antibody and the anti-nuclear antibody test results were all negative. C3, C4, and CH50 levels were 137 mg/dL (137 g/L), 53 mg/dL (53 g/L), and 54.7 U/mL respectively. Haptoglobin level was 191 mg/dL (1.91 g/L). A disintegrin and metalloprotease with thrombospondin-1-like domain (ADAMTS13) activity were 59% (within normal range). Serum TNF-α and IL-6 were high at 16 pg/mL (normal <5) and 44.4 pg/mL (normal <4), respectively. Hepatitis C antibody and hepatitis B surface antigen were negative. Acute viral infection studies of influenza A and B virus, HIV, herpes simplex virus, and cytomegalovirus were all negative. EBV studies showed acute EBV infection: viral capsid antigen (VCA)-IgM was 2.7 (enzyme immunoassay; negative <0.5); VCA-IgG was 0.4 (enzyme immunoassay; negative <0.5). There was no abnormality in chest radiograph or electrocardiogram. Abdominal ultrasound examination showed hepatosplenomegaly, para-aorta lymph nodes, and normal kidney conformation. The clinical course is illustrated in . A bone marrow biopsy was performed on hospital day 5. Hypocellularity with fewer megakaryocytes and many macrophage-phagocytosing erythrocytes and platelets () were revealed. Based on the Imashuku criteria,Citation2 we made a diagnosis of HPS from the clinico-pathological findings. It was concluded that she had developed acute EBV infection-associated HPS. Although sufficient intravenous fluid was administered for AKI, she developed complete anuria on hospital day 4 and hemodialysis was required. The blood purification method was converted to hemodiafiltration due to hypotension. Despite daily blood purification, her renal function did not improve. At first, thrombotic thrombocytopenic purpura was suspected and steroid pulse therapy (1 g/day methylprednisolone intravenously for 3 days) and plasma exchange (2.4 L of freshly frozen plasma as replacement fluid) were performed, then a combination of cyclosporine (150 mg/day) and etoposide (150 mg/day) were also given after the diagnosis of EBV-HPS. Despite the multiple combination therapy, subarachnoidal and intracerebral hemorrhage due to severe thrombocytopenia developed on hospital day 8. This was complicated by respiratory arrest on hospital day 9, requiring mechanical ventilation and hemodiafiltration until her death 19 days later. Under these circumstances, peripheral blood oxygen saturation was kept over 95% and systemic blood pressure was kept around 100 mmHg with continuous dopamine administration (24 μg/kg/min). On hospital day 27 she died. Renal necropsy showed that there were intact glomeruli, almost no acute tubular necrosis, although detachment of tubular cells due to postmortem change were seen, some cell infiltration, and no thrombi. It was characterized by remarkable interstitial edema and some protein casts (). During the clinical course she was always febrile and even just before her death, serum IL-6 and soluble IL-2R were very high at 1960 pg/mL and 4300 U/mL (normal 145 to 519 U/mL), respectively.
Figure 1. Clinical course of the patient. Notes: She developed complete anuria on hospital day 4. The multiple combination therapy, including steroid, cyclosporine, etoposide, and plasmapheresis, failed. She was always febrile over 37 °C and even just before her death, the level of serum IL-6 and soluble IL-2R were very high. Abbreviations: PSL = prednisolone, m-PSL = methylprednisolone sodium-succinate, HD = hemodialysis, HDF = hemodiafiltration, PE = plasma exchange, BT = body temperature, SAH = subarachnoid hemorrhage, n.d. = not data.
Figure 2. (a) Wright–Giemsa staining of the bone marrow aspirate. There are two macrophage-phagocytosing erythrocytes and platelets (original magnification ×400). (b) Periodic acid-Schiff staining of renal necropsy specimen. There is some cell infiltration in the interstitium and no acute tubular necrosis, although detachment of tubular cells due to postmortem changes was detected. There is remarkable edema in the interstitium and some protein casts with intact glomeruli (original magnification ×100).
Discussion
Most previously healthy patients with acute EBV infection recover uneventfully. Patient with acute EBV infection occasionally develop HPS and the prognosis of EBV-HPS is poor.Citation4 Primary HPS is also known as hemophagocytic lymphohistiocytosis (HLH) in the pediatric department. The most effective approach to treating HLH remains unclear, however, a combination of etoposide, dexamethasone, and cyclosporine has been recommended in the HLH-2004 protocol.Citation5 In young adult patients, Imashuku et al. reported that treatment with etoposide within four weeks of diagnosis was extremely effective in improving the outcome of EBV-HLH.Citation6 In the current case, we could not control hypercytokinemia despite the multiple combination therapy.
AKI in EBV infection is uncommon, with an incidence of approximately 1.6% to 4.8% of EBV infection.Citation7 The causes of AKI in EBV infection include rhabdomyolysis, hyperuricemia, hemolysis, nephrotoxic drugs, dehydration, shock, hepato-renal syndrome, and tubulo-interstitial nephritis (TIN).Citation8 The pathogenesis of EBV-associated TIN remains unclear, however, Mayer et al. have suggested that T-lymphocytes of the cytotoxic/suppressor subset infiltrated the kidney.Citation8 There is some literature from Japan, in which inflammatory cell infiltration in a kidney autopsy specimen has been reported.Citation9,Citation10 In the current case, the cause of AKI in the first hospital days may have been predominantly, pre-renal factors because of the high fever and dehydration, however, she developed complete anuria despite sufficient fluid replacement. She developed subarachnoid and intracerebral hemorrhage, and thereafter her blood pressure was dependent on dopamine. High-volume administration of dopamine (24 μg/kg/min) was required to maintain systemic blood pressure. The renal vasoconstriction due to high dose dopamine, resulting in a decrease in renal blood flow, may have led to her persistent renal failure. Renal necropsy revealed intact glomeruli, some cellular infiltration in the interstitium, and almost no acute tubular necrosis. Although she presented complete anuria for about a month, it was very interesting that there were almost no remarkable findings other than interstitial edema and some protein casts. It is known that the histopathologic findings of AKI are inconsistent, are limited to the tubulo-interstitium and are often subtle despite profound dysfunction.Citation11 Heyman et al. suggested that “the limited and focal nature of human acute tubular necrosis markedly contrasts with a prominent decline in glomerular filtration rate, indicating a central role for altered glomerular hemodynamics, possibly related to a tubulo-glomerular feedback signal”.Citation12 Heyman’s hypothesis may explain the discrepancy between renal histology and function in our case.
Holt et al. reported two cases of renal failure complicated by T-cell lymphoma-associated HPS.Citation13 In those cases, renal failure was due to an acute tubular necrosis-like lesion with severe interstitial edema, but in the absence of either glomerular pathology or cellular infiltration. They suggested that hypercytokinemia may have been a direct cause of renal failure and described these renal findings as “cytokine nephropathy”. Supporting this hypothesis, Gauvin et al. suggested that reactive HPS may be an integral part of multiple organ dysfunction syndrome (MODS).Citation14 However, renal tissue findings were not specified in this literature.Citation14 It was reported that common renal tissue findings on the autopsy of eight patients with MODS complicated by AKI were acute TIN.Citation15 Our patient presented MODS and hypercytokinemia. Renal necropsy findings were compatible with that of AKI in MODS and they could be described as “cytokine nephropathy”.
In conclusion, we suggest the following hypothesis for the explanation of the discrepancy of renal histology and function in our patient – hypercytokinemia caused interstitial edema and microcirculatory changes in the kidneys. The renal blood flow was sufficient to prevent tubular necrosis, but not enough to maintain the glomerular filtration rate. This was likely accomplished by so-called corticomedullary blood redistribution and tubulo-glomerular feedback, leading to preservation of medullary pO2. It is important for us to control hypercytokinemia in order to prevent HPS from developing MODS. We suggest that hypercytokinemia may play an important role in the pathophysiology in AKI of EBV-HPS.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Osugi Y, Hara J, Tagawa S, et al. Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood. 1997;89:4100–4103
- Imashuku S. Differential diagnosis of hemophagocytic syndrome: underlying disorders and selection of the most effective treatment. Int J Hematol. 1997;66:135–151
- Sawhney S, Woo P, Murray KJ. Macrophage activation syndrome: a potentially fatal complication of rheumatic disorders. Arch Dis Child. 2001;85:421–426
- Okano M, Gross TG. Epstein--Barr virus-associated hemophagocytic syndrome and fatal infectious mononucleosis. Am J Hematol. 1996;53:111–115
- Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–131
- Imashuku S, Kuriyama K, Sakai R, et al. Treatment of Epstein--Barr virus-associated hemophagocytic lymphohistiocytosis (EBV-HLH) in young adults: a report from the HLH study center. Med Pediatr Oncol. 2003;41:103–109
- Tsai JD, Lee HC, Lin CC, Liang DC, Chen SH, Huang FY. Epstein--Barr virus-associated acute renal failure: diagnosis, treatment, and follow-up. Pediatr Nephrol. 2003;18:667–674
- Mayer HB, Wanke CA, Williams M, Crosson AW, Federman M, Hammer SM. Epstein--Barr virus-induced infectious mononucleosis complicated by acute renal failure: case report and review. Clin Infect Dis. 1996;22:1009–1018
- Mori M, Kurozumi H, Okuyama N, Akagi K. Two cases of fatal virus-associated hemophagocytic syndrome. Jpn J Clin Immunol. 1993;16:182–190 (Japanese)
- Sato S, Kawashima H, Oshiro H, et al. Virological and immunological characteristics of a 19-year-old Japanese female with fatal outcome with Epstein--Barr virus-associated hemophagocytic syndrome. J Clin Virol. 2004;31:235–238
- Rosen S, Stillman IE. Acute tubular necrosis is a syndrome of physiologic and pathologic dissociation. J Am Soc Nephrol. 2008;19:871–875
- Heyman SN, Rosenberger C, Rosen S. Experimental ischemia -- reperfusion: biases and myths -- the proximal vs. distal hypoxic tubular injury debate revisited. Kidney Int. 2010;77:9–16
- Holt S, Varghese Z, Jarmulowicz M, et al. Cytokine nephropathy and multi-organ dysfunction in lymphoma. Nephrol Dial Transplant. 1998;13:1853–1857
- Gauvin F, Toledano B, Champagne J, Lacroix J. Reactive hemophagocytic syndrome presenting as a component of multiple organ dysfunction syndrome. Crit Care Med. 2000;28:3341–3345
- Soejima A, Miyake N, Matsuzawa N, et al. Clinical characterization of acute renal failure in multiple organ dysfunction syndrome. Clin Exp Nephrol. 1998;2:142–150