Abstract
Cisplatin is a popular anticancer drug, but its side effects like nephrotoxicity and hepatotoxicity due to oxidative stress limited its clinical use. In tis study, nephoprotective effect of fractions of Leea asiatica (Leeaceae) leaves was assessed against cisplatin induced toxicity in rats. Leaves of L. asiatica extracted with methanol, ethyl acetate, petroleum ether, and evaluated for in vitro and ex vivo antioxidant activity using several assay models. Methanol extract showed better antioxidant effects, and contain higher amount of phenolic (77.75 ± 0.87 mg GAE/g of dry material) and flavonoid compound (60.98 ± 0.58 mg QE/g of dry material) compared with other extracts. Hance methanol extract was selected for further investigation and fractionated with methanol, ethyl acetate, petroleum ether. Protective effect of methanol extract and its fractions was evaluated against cisplatin (20 mg/kg, i.p.) induced nephrotoxicity. Pretreatment with methanol extract (150 and 300 mg/kg), and its fractions especially methanol, ethyl acetate fraction (75 and 150 mg/kg) significantly reduced blood urea nitrogen, serum creatinine, uric acid levels, and decreased malondialdehyde level and increase total protein and albumin level (p < 0.05, 0.01). Ethyl acetate fraction produced highest nephroprotective, possibly by inhibiting lipid peroxidation process. Result suggested that ethyl acetate fraction possesses potent nephroprotective activity and can be used an adjunct therapy aiming to improve the effectiveness of several nephrotoxic drugs.
Introduction
Kidneys are dynamic organs and play imperative role in maintaining body homeostasis, affected by many chemicals and drugs. Because of its unique metabolism, the kidney is a one of the primary target of the toxic effects of drugs, xenobiotics, and oxidative stress.Citation1 Cisplatin [cis-diamminedichloroplatinum (II)] is a most potent and effective chemotherapeutic agent against a diverse spectrum of malignancies. However, the use of cisplatin in combating cancer is limited by the development of numerous reversible and irreversible side effects including nephrotoxicity, neurotoxicity, bone marrow toxicity, gastrointestinal toxicity and ototoxicity.Citation2,Citation3
Natural products and their active principles as sources for new drug discovery and treatment of diseases have attracted considerable attention of researchers. Medicinal plants contain a wide variety of natural antioxidants such as phenolic acids, flavonoids and tannins, which proved their potential role in prevention and treatment of diseases.Citation4,Citation5 The plants of genus Leea (Leeaceae) are widely distributed in tropical and subtropical countries, and have long been extensively used in folk medicine. The species Leea possess different biological and antioxidant activities. Several antioxidant compounds also isolated from the plants under genus Leea.Citation6,Citation7 Leea asiatica (L.) Ridsdale. a folk medicinal plant of India used in the treatment of a broad spectrum of diseases including worm infection, wound, eye diseases, bone fracture, diabetes and gastrointestinal disorders.Citation8–Citation10 This study was undertaken to examine protective effect of the leaves of Leea asiatica against cisplatin induced nephrotoxicity.
Materials and methods
Plant materials and extraction procedure
Leaves of Leea asiatica (L.) Ridsdale. were collected from Tripura, India and identified by Dr. BK Datta, Department of Botany, Tripura University, Tripura, India. A voucher specimen (TU/BOT/HEB/SS23072011c) has been kept in the herbarium of Plant Taxonomy & Biodiversity Laboratory, Tripura University.
The fine powder of dried plant material was extracted with methanol using Soxhlet apparatus. The filtrate was concentrated and solvent was evaporated to dryness to obtain methanol extract of L. asiatica (MELA). Similar protocol was repeated with ethyl acetate and petroleum ether to obtain crude L. asiatica ethyl acetate extract (EELA) and petroleum ether extract (PELA).
Experimental animals
Healthy Albino mice (20–30 g) were used for the acute toxicity study and nephroprotective activity, while Wistar rats (150–200 g) were used for the ex vivo antioxidant studies. Animals were maintained under controlled environmental conditions. The study was approved by the Institutional Animal Ethical Committee (Reg. No. 1305/ac/09/CPCSEA).
Scheme of the study
The study was performed in three stages as follows:
First stage – to evaluate antioxidant effect of extracts and to select an extract based on antioxidant activity.
Second stage – to evaluated nephroprotective activity of selected extract.
Third stage – to evaluated nephroprotective activity of fractions of selected extract (if selected extract shows positive activity).
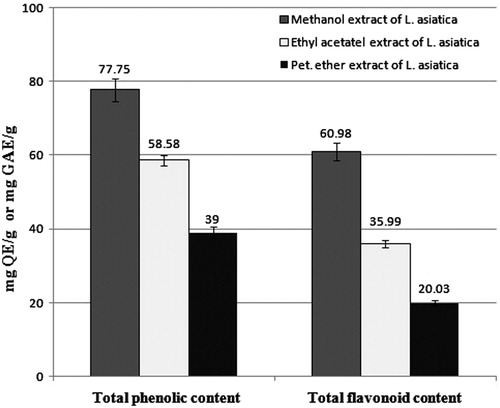
Total phenolic and total flavonoid content in extracts
Total phenolic content in the extracts was determined by Folin–Ciocalteu method. Results were expressed in milligram gallic acid equivalents per gram (mg GAE/g) of dry sample.Citation11 Total flavonoid content of extracts was estimated by aluminium nitrate-potassium acetate reagent method following the method of Asokkumar et al. and expressed as milligram quercetin equivalents per gram (mg QE/g) of dry sample.Citation12
In vitro and ex vivo antioxidant activity of extracts
The free radical scavenging activity of extracts of L. asiatica leaf was determined by several in vitro assays include 2,2-diphenyl-1-picryl-hydrazy radical (DPPH•),Citation13 superoxide anion radical (),Citation14 hydroxyl radical (OH•),Citation15 nitric oxide radical (NO•),Citation16 hydrogen peroxide (H2O2) scavenging activity,Citation17 reducing power ability,Citation18 metal chelating ability,Citation19 and total antioxidant activity.Citation20
Rat liver homogenate (5%) was used to perform lipid peroxidation inhibition assay, rutin was used as standard. Thiobarbituric acid (TBA) reacts with malondialdehyde (MDA) to produce a pink chromogen, which was detected spectrophotometrically at 532 nm.Citation21 Oxidative hemolysis inhibition assay was performed to find the protective effect of extracts on erythrocytes against H2O2 induced hemolysis, which was evaluated by using 0.5% erythrocyte suspension prepared from rat blood.Citation22 All tests were performed in triplicate and percentage scavenging activity of extracts was calculated.
Selection and chromatographic of fractionation of potent extract
Antioxidant study of extract revealed MELA possess better free radical scavenging effect compare to other extracts. Oxidative stress is one of the foremost underlying causes of nephrotoxicity. Thus MELA was selected further study (nephroprotective activity).
MELA was fractionated by column chromatography. MELA was solubilized in minimum volume of solvent and pre-absorbed onto silica gel (60–120 mesh), and fractionated with column chromatographic technique using different solvents in the order of increasing polarity viz petroleum ether, ethyl acetate and methanol to obtain petroleum ether fraction (PFLA), ethyl acetate fraction (EFLA) and methanol fraction (MFLA) of L. asiatica.
Acute toxicity study
The fixed dose (OCED Guideline no. 423, Annexure 2 d) method of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) was used for toxicity studies. The tested extract and fractions were administrated orally.Citation23
Nephroprotective activity
Nephroprotective effect of MELA and its fractions (PFLA, EFLA, MFLA) was evaluated against cisplatin induced nephrotoxicity. At first nephroprotective activity of MELA was evaluated, and after confirmation of nephroprotective activity of MELA the protective effect of PFLA, EFLA, MFLA were also evaluated. All the test samples, standard drug (rutin) and vehicle were administered for six consecutive days once daily by oral route. On fourth day, single intraperitoneal injection of cisplatin (20 mg/kg) was given to induced nephrotoxicity in mice.Citation24 The mice were sacrificed 72 h following cisplatin administration and blood samples were collected for estimation of different biochemical parameters.
Serum was used to estimate blood urea nitrogen (BUN), creatinine levels, uric acid, total protein, albumin by commercially available kits. Malondialdehyde (MDA) level was also determined.
Statistical analysis
The results are expressed as mean ± S.E.M. Statistical difference was tested by using one-way analysis of variance followed by Tukey tests. A level of p < 0.05 was used as the criterion for statistical significance.
Results
Total phenolic and total flavonoid content
Among the three extracts MELA contain highest amount of total phenolic and total flavonoid content. The total phenolic content and total flavonoid content of MELA was 77.75 ± 0.87 mg GAE/g and 60.98 ± 0.58 mg QE/g of dry material ().
In vitro and ex vivo antioxidant activity
Extracts shows concentration dependent radical scavenging and antioxidant activity. The IC50 value of extracts tested against different methods were given in . At 48 µg/mL, MELA resulted 64.13% scavenging effect, while ascorbic acid (16 µg/mL) possessed 91.17% scavenging activity. The extracts (20–160 µg/mL) effectively quench the superoxide anion. IC50 value of MELA, EELA, PELA and BHA on superoxide scavenging activity was 29.9 ± 0.69, 41.0 ± 0.91, 53.4 ± 1.13 and 23.8 ± 0.89 µg/mL respectively.
Table 1. Antioxidant activities of the extracts of L. asiatica leaf by using different in vitro models.
The degradation of deoxyribose by Fe+3-ascorbic acid-EDTA-H2O2 system was significantly decreased by extracts and quercetin, proving the significant (p < 0.05) OH• scavenging activity of extracts. MELA possess almost similar activity (IC50 = 26.5 ± 0.81 µg/mL) like that of quercetin (IC50 = 20.8 ± 0.78 µg/mL). MELA also showed excellent NO• scavenging activity, which was better than the ascorbic acid. At a concentration of 120 µg/mL, NO• scavenging activity of MELA and ascorbic acid was 80.33% and 78.93%, respectively.
Extracts (25–400 µg/mL) were moderately neutralize H2O2. IC50 value of MELA and EELA was 118.6 ± 1.50, 155.5 ± 2.07 µg/mL, respectively. Extracts and α-tocopherol interfered with the formation of ferrous and ferrozine complex, suggesting that extracts possess significant ferrous ion chelating activity. MELA (IC50 = 114.6 ± 1.40 µg/mL) had similar chelating activity with that of standard (IC50 = 107.0 ± 1.23 µg/mL).
Leea asiatica leaf extracts inhibit MDA formation in liver tissue homogenates significantly. At 120 µg/mL, both MELA and rutin had almost similar activity, but in 160 µg/mL MELA produced 82.10%, while rutin produced 91.53% inhibition. Erythrocyte hemolysis was effectively inhibited by the extracts (20–160 µg/mL). IC50 value of ascorbic acid, MELA, EELA, PELA were 45.3 ± 0.08, 55.1 ± 0.09, 59.7 ± 0.10 and 148.9 ± 0.18 µg/mL, respectively.
The extracts showed the concentration-dependent reducing powers ability. The sequence for reducing power was ascorbic acid >MELA >EELA >PELA. The extracts also demonstrated significant total antioxidant effect evident by the preventing the peroxidation of linoleic acid. On 9th day absorbance value of control was decreased suggested that the formation of peroxides was stopped because of non-availability of linoleic acid. Both MELA and EELA showed better activity than the α-tocopherol observed during the incubation time.
MELA showed better antioxidant activity compare with EELA and PELA. A potent free radical scavenger may exert beneficial and protective effect against nephrotoxicity, therefore, MELA was selected fractionation and evaluation of nephroprotective activity.
Acute toxicity study
In acute toxicity study of MELA and its fractions (PFLA, EFLA, MFLA) did not show mortality at the dose of 2000 mg/kg. Therefore, 2000 mg/kg dose was considered as ALD50 cut off the dose under Globally Harmonized Classification System (GHS) category 5 (safe dose), as per OECD guideline 423 (Annexure 2 d). Common side effects were not recorded within the 7 d of observation.
Nephroprotective activity of methanol extract of L. asiatica (MELA)
Cisplatin induced negative control group showed marked increase in BUN, creatinine, uric acid level, while significant decrease in total protein and albumin level when compared with normal control group. Treatment with MELA (150 and 300 mg/kg) significantly (p < 0.01, 0.001) reduced BUN, creatinine, uric acid, and increased total protein and albumin level. Higher dose of MELA was more effective, though lower dose of MELA also produced significant effect (except elevation of albumin level) but less than the standard ().
Table 2. Naphroprotective activity of methanol extracts of L. asiatica leaf.
In untreated negative control group, serum MDA level was 5.19 ± 0.22 nM/min/mg protein. The two used doses of MELA (150 and 300 mg/kg) reduced liver tissue MDA level by 38.2% and 53.0%, respectively.
Nephroprotective activity of fractions of MELA
MELA showed significant nephroprotective activity therefore protective effect of different fractions of MELA at a dose of 75 and 150 mg/kg were evaluated against cisplatin induced nephrotoxicity. MFLA and EFLA showed significant nephroprotective activity determine by estimation of different biochemical parameters. Level of BUN, creatinine, uric acid were significantly decreased and level of total protein, albumin were increased when compare with negative control. PFLA fails to produce any significant effect on serum parameter at lower and higher dose. Higher dose of EFLA showed highest activity and almost similar to that of rutin. Administration of EFLA (150 mg/kg/day) produced 67.9, 64.0, 61.1% decrease in BUN, creatinine, uric acid level, and 65.1, 58.0% increase in total protein and albumin level in compare with negative control group. The raised serum MDA after cisplatin administration was also restored by the MFLA (150 mg/kg) and EFLA (150 mg/kg) by 44.4% and 48.8%, respectively ().
Table 3. Protective effect of fractions of L. asiatica leaf methanol extract against cisplatin induced nephrotoxicity.
Discussion
The present study reports the free radical scavenging activity, nephroprotective effect of Leea asiatica leaves for the first time. Nephrotoxicity is an unwanted side effect of chemotherapy in general. Cisplatin accumulates in mitochondria of renal epithelial cells and induces reactive oxygen species (ROS) such as and OH•, inhibit the activity of antioxidant enzymes in renal tissue and responsible for depletion of intracellular concentrations of glutathione (GSH).Citation25,Citation26 Cisplatin is linked with an increase in lipid peroxidation in the kidney tissues that leads to nephrotoxicity.Citation2 Recently, attention is focused on the development of antioxidants from natural sources that are able to ameliorate cisplatin induced nephrotoxicities.
Our investigation showed that leaf extracts of L. asiatica particularly methanol extract is a potent free radical scavenger. Extract significantly scavenged DPPH• that may relate with the inhibition of lipid peroxidation. The and OH• are the most reactive and strongest ROS in living system responsible for enormous biological damage through lipid peroxidation and nephrotoxicity.Citation27,Citation28 NO• generated by the phagocytes and endothelial cells, to yield more reactive species like peroxynitrite which can be decomposed to produce OH•.Citation14,Citation27 MELA showed significant
, OH•, NO•, H2O2 scavenging and metal ion chelating effect which indicates the possible beneficial effect of extract in preventing oxidative stress induced diseases.
Lipid peroxidation can induce disturbance and alteration of biological membranes, disruption membrane transport proteins and deactivation of membrane-associated enzymes, generates potentially toxic products, and therefore may responsible for wide variety of diseases.Citation21,Citation29 Results suggested that MELA have protective effect against cell membrane lipid oxidation, and provides significant protection to erythrocytes against oxidative damage. MELA contain highest polyphenol, flavonoid compound which may responsible for its potent antioxidant and radical scavenging activity.
In this present study, MELA showed better antioxidant activity and role of oxidative stress in the pathogenesis of cisplatin induced nephrotoxicity well recognized, therefore only MELA was evaluated for nephroprotective activity. Investigation in first stage showed that MELA process significant nephroprotective activity, thus further investigation was carried out to find the most beneficial fraction of MELA against cisplatin induced nephrotoxicity. Urea is the principal nitrogen-containing product of protein metabolism, while uric acid is considered as the key metabolic product of purine nucleotides, adenosine and guanosine.Citation30 BUN is consequent of diet or tissue sources, found in the liver protein that is usually excreted in the urine. In renal disorder, the production rate of serum urea exceeds the renal clearance rate; hence urea accumulates in serum.Citation31 Urea and creatinine level in serum is considered as indicators of renal function. Creatinine is generally derived from endogenous sources by breakdown of tissue creatinine. Augmented urea level in blood/serum indicates the increased protein catabolism and increased synthesis of arginase in mammals causes the conversion of ammonia to urea. Total protein levels including albumin levels reduced in hepatotoxic/nephrotoxic conditions resulted from the faulty protein biosynthesis.Citation30,Citation31 Cisplatin administration produced a significant increase in serum ALP, creatinine, BUN, and uric accompanied by significant decrease in total proteins and albumin that reflect its interaction with cell membrane, leading to altered cell membrane permeability, and alteration of functional integrity in the kidney. In contrast, MFLA and EFLA treated mice showed significant ameliorate these markers, thus showing their ability to protect against cisplatin-induced kidney.
In the present study, single dose of cisplatin resulted in renal lipid peroxidation, determined by the increase in the MDA content when compared to normal control group. These data are supported by previous studies where cisplatin induced lipid peroxidation and damage in the renal tissues of rats was reported.Citation32 Pretreatment with methanol extract, MFLA, EFLA prevented the increase in MDA in kidney tissue. In resent investigation we also demonstrated that ethyl acetate fraction of MELA showed significant DPPH•, NO• scavenging, inhibition of lipid peroxidation and total antioxidant capacity.Citation33 The potent free radical scavenging activity of EFLA may be one of the underlying mechanism for its nephroprotective activity and fractions may exert a protective effect is by acting as an antioxidant preventing kidney damage caused by ROS formed from cisplatin.
In conclusion, the results of the present studies indicate that the ethyl acetate fraction of methanol extract of L. asiatica leaves possesses profound nephroprotective activity; possibly by inhibiting lipid peroxidation process. Further investigation of these promising protective effects of EFLA against cisplatin-induced nephrotoxicity may have a significant impact on developing clinically feasible strategies or new chemical entity to treat patients with renal failure, or as an adjunct therapy aiming to improve the effectiveness of several nephrotoxic drugs.
Declaration of interest
The authors declare that they have no conflicts of interest to disclose. This research received no specific grant from any funding agency in the public, commercial, or not for profit sectors.
References
- Ajith TA, Usha S, Nivitha V. Ascorbic acid and α-tocopherol protect anticancer drug cisplatin induced nephrotoxicity in mice: a comparative study. Clinical Chimica Acta. 2007;375:82–86
- Nitha B, Janardhanan KK. Aqueous-ethanolic extract of morel mushroom mycelium Morchella esculenta, protects cisplatin and gentamicin induced nephrotoxicity in mice. Food Chem Toxicol. 2008;46:3193–3199
- Khan SA, Priyamvada S, Khan W, Khan S, Farooq N, Yusufi ANK. Studies on the protective effect of green tea against cisplatin induced nephrotoxicity. Pharmacol Res. 2009;60:382–391
- Ajith TA, Aswathy MS, Hema U. Protective effect of Zingiber officinale roscoe against anticancer drug doxorubicin-induced acute nephrotoxicity. Food Chem Toxicol 2008;46:3178–3181
- Sen S, Chakraborty R. The role of antioxidants in human health. In: Silvana A, Hepel M, eds. Oxidative Stress: Diagnostics, Prevention, and Therapy. Washington, DC: American Chemical Society; 2011:1–37
- Beck POD, Dijoux M, Cartier G, Mariotee A. Quercitrin 3′-sulphate from leaves of Leea guinensis. Phytochemistry. 1998;47:1171–1173
- Saenjum C, Kadchumsang S, Chansakaow S, Suttajit M, Chaiyasut, C. Screening of lanna medicinal plants with anti-inflammatory property assessed by free radical scavenging activities. Asian J Pharm Sci. 2007;3:65–73
- Bhandary MJ, Chandrashekar KR, Kaveriappa KM. Medical ethnobotany of the Siddis of Uttara Kannada district, Karnataka, India. J Ethnopharmacol. 1995;47:149–158
- Prasad PRC, Reddy CS, Raza SH, Dutt CBS. Folklore medicinal plants of North Andaman Islands, India. Fitoterapia. 2008;79:458–464
- Sen S, Chakraborty R, De B, Devanna N. An ethnobotanical survey of medicinal plants used by ethnic people in West and South district of Tripura, India. J Forestry Res. 2011;22:417–426
- Aiyegoro OA, Okoh AI. Preliminary phytochemical screening and in vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement Alternat Med. 2001;10:21
- Asokkumar K, Umamaheswari M, Baharudeen A, Sivashanmugam AT, Subhadradevi V, Ravi TK. Antioxidant and hepatoprotective activities of the fractions of Ficus microcarpa using in vitro and ex vivo models. Functional Plant Sci Biotechnol. 2010;4(suppl. 1):17–27
- Harlalka GV, Patil CR, Patil MR. Protective effect of Kalanchoe pinnata pers. (Cressulaceae) on gentamycin-induced nephrotoxicity in rats. Indian J Pharmacol. 2007;39:201–205
- Nagulendran KR, Velavan S, Mahesh R, Begum VH. In vitro antioxidant activity and total polyphenolic content of Cyperus rotundus rhizomes. E-J Chem. 2007;4:440–449
- Ozyurek M, Bektasoglu B, Guclu K, Apak R. Hydroxyl radical scavenging assay of phenolics and flavonoids with a modified cupric reducing antioxidant capacity (CUPRAC) method using catalase for hydrogen peroxide degradation. Analytical Chimica Acta. 2008;616:196–206
- Yen G, Lai H, Chou H. Nitric oxide-scavenging and antioxidant effects of Uraria crinita root. Food Chem. 2001;74:471–478
- Bozin B, Mimica-Duki N, Samojlik I, Goran A, Igic R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chem. 2008;111:925–929
- Pal J, Ganguli S, Tahsin KS, Acharya K. In vitro free radical scavenging activity of wild edible mushroom Pleurotus squarrosulus (Mont.) Singer. Indian J Experimental Biol. 2010;47:1210–1218
- Gulcin I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology. 2006;217:213–220
- Liu Q, Yao H. Antioxidant activities of barley seeds extracts. Food Chem. 2007;102:732–737
- Tai Z, Cai L, Dai L, et al. Antioxidant activity and chemical constituents of edible flower of Sophora viciifolia. Food Chem. 2011;126:1648–1654
- Coulibaly AY, Sombie PAED, Tibiri A, Kiendrebeogo M, Compaore MMY, Nacoulma OG. Protective effect of Scoparia dulcis on brain and erythrocytes. Curr Res J Biol Sci. 2011;3:254–261
- Veerarghavan P. Committee for the Purpose of control and supervision of Experiments on Animals (CPCSEA), Animal Welfare Division, Government of India (Guideline No. 423, Annexure-2d of OECD). 2001
- Fouad AA, Morsy MA, Gomaa W. Protective effect of carnosine against cisplatin-induced nephrotoxicity in mice. Environ Toxicol Pharmacol. 2008;2:292–297
- Huang Q, Dunn RT, Jayadev S, et al. Assessment of cisplatin induced nephrotoxicity by microarray technology. Toxicol Sci. 2001;63:196–207
- Liu J, Liu Y, Habeebu SM, Klaassen CD. Metal-lothionein MT. Null mice are sensitive to cisplatin-induced hepatotoxicity. Toxicol Appl Pharmacol. 1998;149:24–31
- Hazra B, Biswas S, Mandal N. Antioxidant and free radical scavenging activity Spondias pinnata. BMC Complement Alternat Med. 2008;8:63
- Rao KS, Munjuluri PR, Keshar NK. In vitro antioxidant activity and total phenolic content of Mimusops elengi bark. Indian J Pharm Educ Res. 2011;45:317–324
- Niki E. Assessment of antioxidant capacity in vitro and in vivo. Free Radical Biol Med 2010;49:503–515
- Renugadevi J, Prabu SM. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology. 2009; 256:128–134
- Parameshappa B, Basha MSA, Sen S, et al. Acetaminophen-induced nephrotoxicity in rats: protective role of Cardiospermum halicacabum. Pharm Biol. 2012;50:247–253
- Mora LO, Antunes LMG, Francescato HDC, Bianchi MLP. The effects of oral glutamine on cisplatin-induced nephrotoxicity in rats. Pharm Res. 2003;47:517–522
- Sen S, De B, Devanna N, Chakraborty R. Anthelmintic and in vitro antioxidant evaluation of fractions of methanol extract of Leea asiatica leaves. Ancient Sci Life. 2012;31:101--106