Abstract
Apolipoprotein E (apoE), one of the major plasma lipoproteins, plays a major role in the transport and metabolism of lipids by acting as a ligand. apoE gene contains three potential alleles: ϵ2, ϵ3 and ϵ4, forming six genotypes: E2E2, E2E3, E2E4, E3E3, E3E3 and E4E4. Association between apoE gene polymorphism and triglyceride (TG) is still controversial. There was no any meta-analysis to explore the association of apoE gene polymorphism with triglyceride level, and this meta-analysis was performed to evaluate the association between apoE gene polymorphism and triglyceride in patients with renal diseases. A predefined literature search and selection of eligible relevant studies were performed to collect data from electronic databases. Twenty-four articles were identified for the analysis of association between apoE gene polymorphism and triglyceride level. Subjects with E2E3 or E3E4 had a higher TG than those with E3E3. Subjects with ϵ4 had a higher TG than those with ϵ3. Subjects with ϵ2 had a slightly higher TG than those with ϵ3, although there was no statistical difference. Interestingly, subjects with ϵ4 had a much higher TG than those with ϵ2. In conclusion, E2E3, E3E4 or ϵ4 was associated with higher level of TG. However, more studies should be performed in the future.
Introduction
Apolipoprotein E (apoE) is one of the major plasma lipoproteins, and it plays a major role in the transport and metabolism of lipids by acting as a ligand for low density lipoprotein receptors.Citation1 ApoE, a 229-amino-acid polypeptide, is classified into three major isoforms (ϵ2, ϵ3 and ϵ4) according to the differences in amino acids at positions 112 and 158, forming six genotypes: E2E2, E2E3, E2E4, E3E3, E3E3 and E4E4.Citation2,Citation3 ϵ3 and E3E3 are the wild type of apoE, and ϵ2, ϵ4, E2E2, E2E3, E2E4, E3E3 and E4E4 are the mutation type of apoE. The present evidences indicate that apoE is associated with the renal diseases.Citation4–6 It is associated with the metabolism of triglyceride (TG).Citation7 The TG accumulation in kidney is associated with the kidney injury.Citation8
At the presented data, some reports showed that apoE gene polymorphism was associated with the expression of TG. However, there were also some investigations finding that apoE gene polymorphism was not associated with the expression of TG. The available evidence is weak, due to sparseness of data or disagreements among the reported investigations. The evidence from meta-analysis may be powerful when compared with the individual investigations. There was no any meta-analysis performed to investigate the association between the apoE gene polymorphism and the TG level in renal diseases. This meta-analysis was performed to investigate the association of the apoE gene polymorphism with the TG level in patients with renal diseases.
Materials and methods
Search strategy
The relevant studies were screened from the search engines of PubMed, Embase, and Cochrane Library on March 1, 2012. “(apoE AND apolipoprotein E) AND (renal OR kidney)” was used in PubMed, Embase, and Cochrane Library. We also extended search spectrum to the “related articles” and the bibliographies of all retrieved studies. If multiple publications from the same study group using the same data occurred, we only recruited the most complete paper for analysis.
Inclusion and exclusion criteria
Inclusion criteria
(1) The study had to be about renal diseases; (2) providing the detailed gene distribution of apoE; (3) offering the data of level of TG.
Exclusion criteria
(1) Review articles; (2) same data for multiple publications; (3) investigating the association of other genes in renal diseases; (4) investigating the role apoE in diseases.
Data extraction and synthesis
The following information was extracted from each study independently by the investigators: first author’s surname, year of publication, location of study, disease type, level of TG, and the number of subjects. The results were compared and disagreements were resolved by discussion.
Statistical analysis
Available data were entered into Cochrane Review Manager (RevMan, Version 5) and analyzed. The pooled statistic was counted using the fixed effects model, but a random effects model was conducted when the p value of heterogeneity test was less than 0.1. Results were expressed with weighted mean differences (WMD) for continuous data, and 95% confidence intervals (CI) were also counted. p < 0.05 was required for the overall OR to be deemed statistically significant. I2 was used to test the heterogeneity between the included studies. The Begg adjusted rank correlation testCitation9 and the Egger regression asymmetry testCitation10 were used for exploring publication bias (p < 0.1 was considered significant), when the number of the included studies was more than 15.
Results
Study characteristics for the relationship between apoE gene polymorphism with TG expression
Twenty-four studies were included into the meta-analysis for the relationship between apoE gene polymorphism and TG expression. Two studiesCitation11,Citation12 were for the compassion of E2E2 versus E3E3. 12 reportsCitation11–22 were recruited into the study of E2E3 versus E3E3 (including 17 comparisons). Three reportsCitation11,Citation12,Citation14 were recruited into the study of E2E4 versus E3E3 (including 4 comparisons). Twelve reportsCitation11–22 were recruited into the study of E3E4 versus E3E3 (including 17 comparisons). Three reportsCitation11,Citation12,Citation14 were recruited into the study of E4E4 versus E3E3 (including 4 comparisons). Two reportsCitation23,Citation24 was included for the meta-analysis of ϵ2 versus non-ϵ2 (including 4 comparisons). One studyCitation25 was recruited into our meta-analysis for the comparison of ϵ4 with non-ϵ4 (including 1 comparison). Eighteen studiesCitation12,Citation13,Citation15–22,Citation26–33 were included into the study of ϵ2 versus ϵ3 (including 29 comparisons). Eighteen studiesCitation12,Citation13,Citation15–22,Citation26–32,Citation34 were included into the study of ϵ4 versus ϵ3 (including 29 comparisons). Seventeen studiesCitation12,Citation13,Citation15–22,Citation26–32 were included into the study of ϵ2 versus ϵ4 (including 27 comparisons).
Association of apoE gene polymorphism with TG expression
Subjects with E2E3 or E3E4 had a higher TG than those with E3E3 ( and ; ). Subjects with ϵ4 had a higher TG than those with ϵ3 ( and ). Subjects with ϵ2 had a slightly higher TG than those with ϵ3, although there was no statistical difference ( and ). Interestingly, subjects with ϵ4 had a much higher TG than those with ϵ2 ( and ). It seemed that E2E3, E3E4 or ϵ4 was associated with higher level of TG. The number of included studies for the comparison was more than 15, and those results might be robust to some extent.
Figure 1. Association of apoE gene polymorphism with triglyceride level using the compassion of E2E3 versus E3E3.
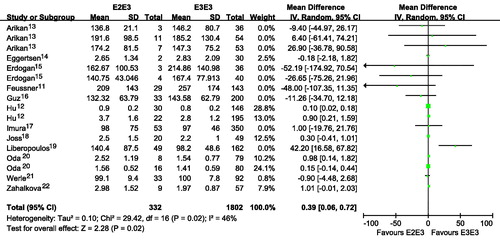
Figure 2. Association of apoE gene polymorphism with triglyceride level using the compassion of E3E4 versus E3E3.
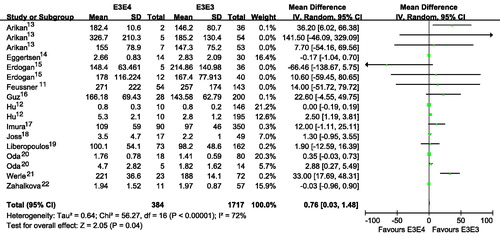
Figure 3. Association of apoE gene polymorphism with triglyceride level using the compassion of ϵ4 versus ϵ3.
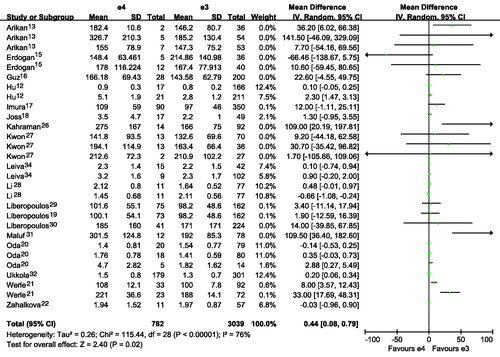
Figure 4. Association of apoE gene polymorphism with triglyceride level using the compassion of ϵ2 versus ϵ3.
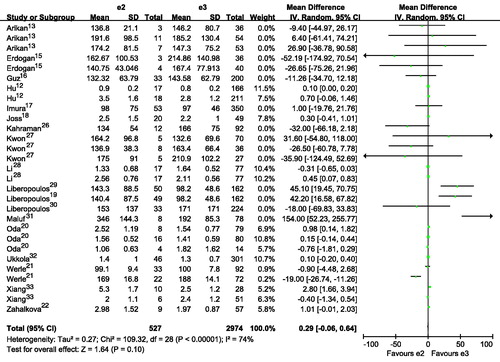
Figure 5. Association of apoE gene polymorphism with triglyceride level using the compassion of ϵ2 versus ϵ4.
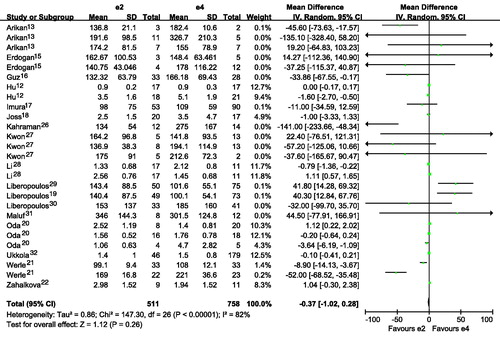
Table 1. Meta analysis of the association of apoE gene polymorphism with triglyceride level.
Subjects with E2E2 or E2E4 had a lower TG level than those with E3E3, and subjects with E4E4 had a higher level of TG than those with E3E3, although there was not statistically different (). Subjects with ϵ2 had a higher level of TG than those with non-ϵ2 (). Furthermore, subjects with ϵ4 had a higher level of TG than those with non-ϵ4, although there was not statistically different (). It seemed that ϵ2 was associated with higher level of TG. However, the number of included studies for those comparisons was less than 15, and more studies should be performed in the future.
Publication bias test for the association of apoE gene polymorphism with TG expression
Publication bias test for the comparisons of E2E3 versus E3E3, E3E4 versus E3E3, ϵ2 versus ϵ3, ϵ4 versus ϵ3, ϵ2 versus ϵ4. There were no publication bias for the comparisons of E2E3 versus E3E3, E3E4 versus E3E3, ϵ2 versus ϵ3, ϵ4 versus ϵ3, ϵ2 versus ϵ4 (E2E3 versus E3E3: Begg p = 0.300, Egger p = 0.215; E3E4 versus E3E3: Begg p = 0.300, Egger p = 0.269; ϵ2 versus ϵ3: Begg p = 0.144, Egger p = 0.190; ϵ4 versus ϵ3: Begg p = 0.922, Egger p = 0.567; ϵ2 versus ϵ4: Begg p = 0.780, Egger p = 0.823).
Discussion
High serum TG level is an important characteristic for many renal diseases, such as NS,Citation35 glomerulonephritis,Citation36 chronic kidney disease with dialysis,Citation37 and so on. The increased level of TG might be an important risk factor for the onset susceptibility of renal diseases. In our study, subjects with E2E3 or E3E4 had a higher TG than those with E3E3. Subjects with ϵ4 had a higher TG than those with ϵ3. It seemed that E2E3 or E3E4 was associated with higher level of TG, and ϵ4 were associated with higher level of TG. There was no publication bias test for the comparisons of E2E3 versus E3E3, E3E4 versus E3E3, and ϵ4 versus ϵ3. The conclusions for those comparisons were robust to some extent. Stiefel et al.Citation38 reported that apoE E3E4 and E4E4 genotypes had a worse lipoprotein profile characterized by higher plasma value of TG. Srivastava et al.Citation39 found that higher TG level was observed in obese subjects with apoE ϵ4 allele than individuals without apoE ϵ4. Ouyang et al.Citation40 found that the level of TG in patients with ϵ4 was higher than those in coronary heart disease patients without ϵ4. The results from those studies mentioned above were similar with our results in this meta-analysis.
The roles of apoE in diseases were also complicated. Many studies reporting that apoE could play a protective role against diseases. apoE can play an antioxidant role.Citation41 apoE has been demonstrated to play an important role in providing protection against mesangial cell injury.Citation42 apoE also appears to be involved in the repair response to tissue injury; for example, markedly increased amounts of apoE are found at sites of peripheral nerve injury and regeneration.Citation43 apoE deficiency in mice leads to the development of atherosclerosis and re-expression of the protein reduces the extent of the disease.Citation44 It seemed that the increased apoE was a protective factor against diseases. However, more studies should be performed to explore the role of apoE in diseases.
In this meta-analysis, we observed that subjects with E2E3 or E3E4 had a higher TG than those with E3E3. Subjects with ϵ4 had a higher TG than those with ϵ3. Subjects with ϵ2 had a slightly higher TG than those with ϵ3, although there was no statistical difference. Subjects with ϵ4 had a much higher TG than those with ϵ2. However, those findings should be regarded cautiously because many other ingredients, such as small sample size of the included report, limited statistical power, heterogeneity of enrolled cases, variable study designs and different interventions, were closely related to affect the results.
In conclusion, E2E3, E3E4 or ϵ4 was associated with higher level of TG. However, more studies should be performed in the future.
Declaration of interest
The authors declare no conflict of interests. The authors alone are responsible for the content and writing of this article.
References
- Zhou TB. Signaling pathways of apoE and its role of gene expression in glomerulus diseases. J Recept Signal Transduct Res. 2013;33(2):73–78
- De Feo E, Cefalo C, Arzani D, et al. A case-control study on the effects of the apolipoprotein E genotypes in nonalcoholic fatty liver disease. Mol Biol Rep. 2012;39(7):7381–7388
- Marrzoq LF, Sharif FA, Abed AA. Relationship between ApoE gene polymorphism and coronary heart disease in Gaza Strip. J Cardiovasc Dis Res. 2011;2(1):29–35
- Zhou TB, Qin YH, Lei FY, Su LN, Zhao YJ, Huang WF. apoE expression in glomerulus and correlation with glomerulosclerosis induced by adriamycin in rats. Ren Fail. 2011;33(3):348–354
- Zhou TB, Qin YH, Lei FY, Su LN, Zhao YJ, Huang WF. Less gelatinases is associated with apolipoprotein E accumulation in glomerulosclerosis rats. Histol Histopathol. 2012;27(2):249–256
- Seshasai RK, Katz R, de Boer IH, et al. Apolipoprotein E and kidney function in older adults. Clin Nephrol. 2012;78(3):174–180
- Brito DD, Fernandes AP, Gomes KB, et al. Apolipoprotein A5-1131T > C polymorphism, but not APOE genotypes, increases susceptibility for dyslipidemia in children and adolescents. Mol Biol Rep. 2011;38(7):4381–4388
- Li S, Nagothu K, Ranganathan G, et al. Reduced kidney lipoprotein lipase and renal tubule triglyceride accumulation in cisplatin-mediated acute kidney injury. Am J Physiol Renal Physiol. 2012;303(3):F437–F448
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315(7109):629–634
- Feussner G, Wey S, Bommer J, Deppermann D, Grutzmacher P, Ziegler R. Apolipoprotein E phenotypes and hyperlipidemia in patients under maintenance hemodialysis. Hum Genet. 1992;88(3):307–312
- Hu P, Qin YH, Lu L, et al. Genetic variation of apolipoprotein E does not contribute to the lipid abnormalities secondary to childhood minimal change nephrotic syndrome. Int Urol Nephrol. 2010;42(2):453–460
- Arikan H, Koc M, Sari H, Tuglular S, Ozener C, Akoglu E. Associations between apolipoprotein E gene polymorphism and plasminogen activator inhibitor-1 and atherogenic lipid profile in dialysis patients. Ren Fail. 2007;29(6):713–719
- Eggertsen G, Heimburger O, Stenvinkel P, Berglund L. Influence of variation at the apolipoprotein E locus on lipid and lipoprotein levels in CAPD patients. Nephrol Dial Transplant. 1997;12(1):141–144
- Erdogan M, Eroglu Z, Biray C, et al. The relationship of the apolipoprotein E gene polymorphism Turkish Type 2 diabetic patients with and without nephropathy. J Endocrinol Invest. 2009;32(3):219–222
- Guz G, Nurhan Ozdemir F, Sezer S, et al. Effect of apolipoprotein E polymorphism on serum lipid, lipoproteins, and atherosclerosis in hemodialysis patients. Am J Kidney Dis. 2000;36(4):826–836
- Imura T, Kimura H, Gejyo F. Apolipoprotein E phenotypes in hemodialysis patients. Kidney Int Suppl. 1999;71:S245–S247
- Joss N, Jardine A, Gaffney D, Boulton-Jones JM. Influence of apolipoprotein E genotype on progression of diabetic nephropathy. Nephron Exp Nephrol. 2005;101(4):e127–e133
- Liberopoulos EN, Miltiadous GA, Athyros VG, et al. Effect of apolipoprotein E polymorphism on serum uric acid levels in healthy subjects. J Investig Med. 2005;53(3):116–122
- Oda H, Yorioka N, Ueda C, Nishida Y, Yamakido M. Apolipoprotein E phenotype and renal disease. Contrib Nephrol. 1997;120:22–29
- Werle E, Fiehn W, Hasslacher C. Apolipoprotein E polymorphism and renal function in German type 1 and type 2 diabetic patients. Diabetes Care. 1998;21(6):994–998
- Zahalkova J, Vaverkova H, Novotny D, Kosatikova Z. Impaired triglyceride tolerance in hemodialysis patients with different apolipoprotein E (apo E) isoforms. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2002;146(2):73–76
- Cardona F, Tinahones FJ, Collantes E, Escudero A, Garcia-Fuentes E, Soriguer FJ. The elevated prevalence of apolipoprotein E2 in patients with gout is associated with reduced renal excretion of urates. Rheumatology (Oxford). 2003;42(3):468–472
- Ma SW, Benzie IF, Yeung VT. Type 2 diabetes mellitus and its renal complications in relation to apolipoprotein E gene polymorphism. Transl Res. 2008;152(3):134–142
- Lim PS, Liu CS, Hong CJ, Wei YH. Prevalence of apolipoprotein E genotypes in ischemic cerebrovascular disease in end-stage renal disease patients. Nephrol Dial Transplant. 1997;12(9):1916–1920
- Kahraman S, Kiykim AA, Altun B, et al. Apolipoprotein E gene polymorphism in renal transplant recipients: effects on lipid metabolism, atherosclerosis and allograft function. Clin Transplant. 2004;18(3):288–294
- Kwon MK, Rhee SY, Chon S, et al. Association between apolipoprotein E genetic polymorphism and the development of diabetic nephropathy in type 2 diabetic patients. Diabetes Res Clin Pract. 2007;77(Suppl 1):S228–S232
- Li HF, Han CF, Wang YX, Lu YS, Zou HQ, Xu QQ. Effect of apolipoprotein E gene polymorphism on serum lipid level before and after renal transplantation. Transplant Proc. 2010;42(7):2513–2517
- Liberopoulos EN, Miltiadous GA, Cariolou M, Kalaitzidis R, Siamopoulos KC, Elisaf MS. Influence of apolipoprotein E polymorphisms on serum creatinine levels and predicted glomerular filtration rate in healthy subjects. Nephrol Dial Transplant. 2004;19(8):2006–2012
- Liberopoulos EN, Miltiadous GA, Cariolou M, Tselepis AD, Siamopoulos KC, Elisaf MS. The influence of serum apolipoprotein E concentration and polymorphism on serum lipid parameters in hemodialysis patients. Am J Kidney Dis. 2004;44(2):300–308
- Maluf DG, Mas VR, Archer KJ, et al. Apolipoprotein E genotypes as predictors of high-risk groups for developing hyperlipidemia in kidney transplant recipients undergoing sirolimus treatment. Transplantation. 2005;80(12):1705–1711
- Ukkola O, Kunnari A, Jokela M, Paivansalo M, Kesaniemi YA. ApoE phenotype is associated with inflammatory markers in middle-aged subjects. Inflamm Res. 2009;58(1):54–59
- Xiang G, Xia B, He Y. The relationship of Apo E2 and renal insufficiency lipid levels in NIDDM. Zhonghua Yi Xue Za Zhi. 1999;79(5):339–341
- Leiva E, Mujica V, Elematore I, et al. Relationship between apolipoprotein E polymorphism and nephropathy in type-2 diabetic patients. Diabetes Res Clin Pract. 2007;78(2):196–201
- Klosterman ES, Moore GE, de Brito Galvao JF, et al. Comparison of signalment, clinicopathologic findings, histologic diagnosis, and prognosis in dogs with glomerular disease with or without nephrotic syndrome. J Vet Intern Med. 2011;25(2):206–214
- Zhou TB, Qin YH, Lei FY, Su LN, Zhao YJ, Huang WF. All-trans retinoic acid regulates the expression of apolipoprotein E in rats with glomerulosclerosis induced by Adriamycin. Exp Mol Pathol. 2011;90(3):287–294
- Marques de Mattos A, Marino LV, Ovidio PP, Jordao AA, Almeida CC, Chiarello PG. Protein oxidative stress and dyslipidemia in dialysis patients. Ther Apher Dial. 2012;16(1):68–74
- Stiefel P, Miranda ML, Bellido LM, et al. Genotype of the CYBA promoter -930A/G, polymorphism C677T of the MTHFR and APOE genotype in patients with hypertensive disorders of pregnancy: an observational study. Med Clin (Barc). 2009;133(17):657–661
- Srivastava N, Achyut BR, Prakash J, Agarwal CG, Pant DC, Mittal B. Association of cholesteryl ester transfer protein (TaqIB) and apolipoprotein E (HhaI) gene variants with obesity. Mol Cell Biochem. 2008;314(1–2):171–177
- Ouyang T, Song JN, Miao Y, et al. Study on relationship between polymorphism of apolipoprotein E gene and syndromes of phlegm and blood stasis in patients with coronary heart disease. Zhong Xi Yi Jie He Xue Bao. 2005;3(6):438–442
- Jofre-Monseny L, Minihane AM, Rimbach G. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol Nutr Food Res. 2008;52(1):131–145
- Arora S, Husain M, Kumar D, et al. Human immunodeficiency virus downregulates podocyte apoE expression. Am J Physiol Renal Physiol. 2009;297(3):F653–F661
- Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240(4852):622–630
- Greenow K, Pearce NJ, Ramji DP. The key role of apolipoprotein E in atherosclerosis. J Mol Med (Berl). 2005;83(5):329–342