Abstract
Background: Chronic kidney disease (CKD) is associated with markedly increased cardiovascular (CV) risk. This increase is not fully explained by traditional CV risk factors but may in part be mediated by nontraditional risk factors, such as inadequate vitamin D (vit D) levels and insulin resistance (IR). Although IR is shown in nondiabetic CKD, its association with vit D deficiency and vascular disease in this population is unknown and what this study aims to investigate. Materials and methods: The study comprised 67 patients with CKD (eGFR ≥ 30 mL/min) and 15 healthy controls matched for age and sex. The phlogosis indexes, vit D levels, IR, carotid intima-media thickness (cIMT), and left ventricular mass index (LVMI) were measured. Results: In our study, the mean value of LVMI and cIMT was significantly higher in patients with eGFR ≥ 30 mL/min compared with controls (p = 0.037 and p < 0.001). The IR and intact parathyroid hormone (iPTH) levels were increased in CKD patients, whereas the serum levels of vit D were significantly reduced (p = 0.044, p = 0.012, p = 0.038). A positive correlation was found between LVMI and IR (r = 0.704, p = 0.041) and a negative correlation was found between IR and vit D levels (r = −0.238, p = 0.031). Conclusions: In our study, IR and vit D deficiency were found to be independent predictors of left ventricular hypertrophy and atherosclerotic disease. Vitamin D deficiency and IR are thus associated with increased CV risk. More novel approaches to improving IR and vit D supplementation in the CKD population might lead to potential strategies for preventing excess CV mortality.
Introduction
Chronic kidney disease (CKD) is an important public health problem with high prevalence, increasing incidence, and very high cardiovascular (CV) morbidity and mortality.Citation1 Traditional risk factors, such as hyperlipidemia and hypertension, fail to fully explain this increased risk,Citation2,Citation3 hence the interest in nontraditional risk factors, such as vitamin D (vit D) deficiency and insulin resistance (IR).Citation4 Identifying and treating risk factors for early CKD may be the best approach to prevent and delay adverse outcome.Citation5
Vitamin D insufficiency or deficiency is widespread in CKD patients and is associated with increased mortality rates in the setting of the disease. Circulating 25-hydroxyvitamin D [25(OH)D] is the hallmark for determining vit D status. Serum parathyroid hormone (PTH) increases progressively when 25(OH)D falls below 75 nmol/L. Concentrations of 25(OH)D below 50 nmol/L, or even below 25 nmol/L, are frequently observed in the general population and remains generally unrecognized and untreated.
Vitamin D receptors (VDRs) are expressed not only in the classical target organs (bone, parathyroid glands, kidneys, and intestine) but also in other nonclassical targets, including the arteries, heart, immune system, endocrine organs, and nervous system. Therefore, the deficiency of active forms of vit D in CKD may explain various abnormalities in biological functions and the survival disadvantage in this disease condition.Citation6,Citation7
Vitamin D is associated with decreased CV-related morbidity and mortality, possibly by modifying the cardiac structure and function; however, firm evidence for either remains lacking.Citation8
Recently, the relationship between CV risk factors and 25(OH)D levels was explored among 15,088 subjects from the NHANES III national cohort registry. In this cross-sectional study, 25(OH)D levels were inversely associated with hypertension, IR, hypertriglyceridemia, obesity, and left ventricular hypertrophy (LVH).Citation9,Citation10 LVH is recognized as one of the strongest risk factors for all-cause and CV mortality in patients with CKD.Citation11
Furthermore, vit D deficiency predisposes to upregulation of the renin–angiotensin–aldosterone system and hypertrophy of both the left ventricle and vascular smooth muscle cells.Citation12
The myocardium is an important target of vit D. VDR knockout mice show myocardial renin overexpression and marked cardiomyocyte hypertrophy. In vitro 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] attenuates cardiomyocyte proliferation and hypertrophy, and treatment with paricalcitol attenuates the development of LVH and LV dysfunction in Dahl rats, a reliable animal model of LVH and vit D deficiency.Citation13 To date, studies in VDR knockout mice represent the most convincing evidence of the fundamental role of vit D in the regulation of LV mass.Citation14,Citation15. Additionally, vit D deficiency causes an increase in PTH, which increases IR and is associated with increased CV risk.Citation16
There is evidence that the vit D and/or PTH axis is important in the pathogenesis of glucose intolerance and IR in patients with CKD. IR is present in the early stages of CKD and has an inverse association with 25(OH)D levels.Citation6,Citation17
IR, described as a subnormal glucose response to a given dose of endogenous or exogenous insulin, has been shown in early nondiabetic CKD. IR is common in patients with mild-to-moderate stage CKD, even when the glomerular filtration rate is within the normal range.Citation17
The predisposing factors responsible for IR in the absence of diabetes mellitus (DM) or obesity in CKD are unknown but are probably related to factors that contribute to vascular disease, such as inflammation and oxidative stress.Citation19 IR, along with oxidative stress and inflammation, also promotes kidney disease by worsening renal hemodynamics through mechanisms such as activation of the sympathetic nervous system, sodium retention, decreased Na+, K+-ATPase activity, and increased GFR.Citation7,Citation20
IR is closely associated with atherosclerosis and CV mortality in the general population. Patients with CKD are known to have IR, advanced atherosclerosis, and a high CV mortality rate.Citation21,Citation22
The relationship of IR with vascular function and inflammation in predialysis, nondiabetic CKD is not known. In patients with end-stage renal disease, IR is an independent predictor of CV disease and is linked to protein energy wasting and malnutrition.Citation23 Nutritional, metabolic, and CV complications of renal disease may be a consequence of abnormal insulin action. Systemic inflammation, oxidative stress, elevated serum adipokine and fetuin-A, metabolic acidosis, vit D deficiency, depressed serum erythropoietin, endoplasmic reticulum stress, and suppressors of cytokine signaling all cause IR by suppressing insulin receptor-PI3K-Akt pathways in CKD.Citation24,Citation25
Aim
This article aims to study the relationship between vit D deficiency, IR, and CV disease markers, such as the carotid intima-media thickness (cIMT) and left ventricular mass index (LVMI), in normoglycemic patients with early CKD.
Materials and methods
Study design and subjects
The study protocol was approved by the Clinical Research Ethics Committee of the Sapienza University of Rome.
Sixty-seven consecutive patients with CKD in stages 1 to 3 (eGFR ≥ 30 mL/min) were enrolled in this study, 27 females and 40 males with a median age of 52.6 years. As control group, we enrolled 15 healthy blood donors matched for age and sex, from the same geographic area of patients, and free of any underlying cardiac or renal disease. shows the patients characteristics. The eGFR was calculated with the abbreviated Modification of Diet in Renal Disease formula, as defined by Levey et al.Citation26
Table 1. Baseline characteristics of patients.
We excluded patients with underlying malignancy, chronic liver disease, chronic obstructive airway disease, systemic lupus erythematosus, chronic rheumatic heart disease, heart failure, congenital heart disease, atrial fibrillation, acute myocardial infarction, valvular heart disease, cerebrovascular disease, common carotid artery stenosis, and acute coronary syndrome within 3 months before the study; patients who refused to give consent; and patients with missing data. In addition, patients with a previous diagnosis of diabetes; current use of oral antidiabetic medication or insulin; or with a history of smoking were excluded. The etiology of CKD in these patients was nephrosclerosis in 19 cases, chronic glomerulonephritis in 14 cases, reflux nephropathy in 8 cases, tubulointerstitial nephritis in 2 patients, chronic pyelonephritis in 4 cases, autosomal polycystic kidney disease in 4 cases, and unknown in 16 cases. Antihypertensive, antiplatelet, and statin therapies were continued in the patients included in the study.
Laboratory measurements
Blood was drawn in the morning after an overnight fast of at least 12 h. In all patients, the levels of fasting plasma glucose, total serum cholesterol, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), creatinine, azotemia, calcemia, phosphate, albumin, serum electrolytes, CPR, and fibrinogen were measured by standard automated techniques. Vitamin D (as 25-hydroxyvitamin D (25-OHD) was measured by radioimmunoassay. PTH was measured by a two-site assay that measures “intact” hormone. Insulin was measured by radioimmunometric assay.
IR was assessed with the homeostasis model assessment (HOMA-IR) originally described by Mathew et al.Citation27
HOMA-IR was calculated with the following formula:
HOMA-IR has a close correlation with the insulin sensitivity index by the standard euglycemic hyperinsulinemic clamp, as shown by Mathew et al. This index can be applied to subjects with renal failure.Citation28
Blood pressure measurements
Blood pressure (BP) was measured with a standard sphygmomanometer and cuffs adapted to the arm circumference after the subject had rested in the supine position for at least 5 min. The systolic and diastolic BP levels were taken as the points of appearance and disappearance of Korotkoff sounds, respectively. The arterial BP levels were measured by a physician three times after a 15-min rest period in the morning, and the mean values for systolic BP (SBP) and diastolic BP (DBP) were calculated for all participants. The average of three measurements was used for analysis. Hypertension was defined as SBP > 140 mmHg or DBP > 90 mmHg on repeated measurements or the use of antihypertensive drugs.
Carotid intima-media thickness assessment
The common and internal carotid artery imaging was done by a single investigator in the vascular laboratory suite under standardized conditions with the high-resolution B-mode ultrasound machine Toshiba Aplio xV (Toshiba American Medical Systems, Inc., Tustin, CA) equipped with a 5–12-MHz linear transducer. All ultrasonic examinations were stored on a super VHS video system (JVC U.S.A., Wayne, NJ) for offline processing. Video images were captured in end diastole triggered by electrocardiographic recording. The images were then digitized and transferred to a personal computer for further analysis. One longitudinal image of the common carotid artery and three longitudinal images of the internal carotid artery were acquired. The mean common and internal carotid IMT was measured with a semiautomated computer analysis system that detects the lumen/intima borderline and the media/adventitia borderline with the use of a gray-value algorithm. The differences between these two borderlines were measured along a line orthogonal to the arterial wall. Single IMT values were obtained from pixel-to-pixel measurements on neighboring lines, perpendicular to the vertical line, and then averaged and expressed as the mean IMT for that segment. The mean IMT was computed as the average IMT on both sides. The images were analyzed by a technician blinded to patient identity and study group.Citation29
Echocardiography
M-mode 2D echocardiographic examinations were done by a single experienced sonographer in the echocardiography laboratory and using a standard institutional protocol. Commercially available instruments (Toshiba Aplio xV, Toshiba American Medical Systems, Inc., Tustin, CA) equipped with 2.25 to 7.5 MHz imaging transducers were used; the subjects were in the left decubitus position, and the sonographer was blinded to all clinical details of the patients.
All echocardiographic data were recorded according to the guidelines of the American Society of Echocardiography (ASE). The end-diastolic and end-systolic left ventricular internal diameter (LVED and LVES, respectively), interventricular septum thickness (IVST), and posterior wall thickness (PWT) were measured. The left ventricular mass (LVM) was estimated by Devereux's formula normalized by body surface area (BSA) and height.Citation30–33
Statistical analysis
The normality of variables was tested by the Kolmogorov–Smirnov method for normal distributions. All continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as number (percentage). A two-tailed Student’s t-test or Mann–Whitney U-test was done to determine the differences between groups. A chi-square test was used for comparison of categorical data. For bivariate correlation, Pearson’s or Spearman’s correlation was used to determine the relationship and the strength of association between variables. Data management and analysis were done with the IBM® SPSS® Statistics 18 software for Windows® (IBM Corporation, Armonk, NY). The significance level was set at 0.05.
Results
presents the general characteristics of the study participants. There were no statistically significant differences in age, sex, height, weight, BMI, serum sodium, potassium, calcium, phosphorus, Ca*P product, glucose, HDL, triglycerides, and C-reactive protein between the groups. The mean value of LVMI was significantly higher in patients with eGFR ≥ 30 mL/min compared with controls (139 ± 19 vs. 104 ± 22, p = 0.037). Patients with CKD had a significantly higher cIMT compared with healthy controls (1.1 ± 0.3 vs. 0.7 ± 0.1, p < 0.001).
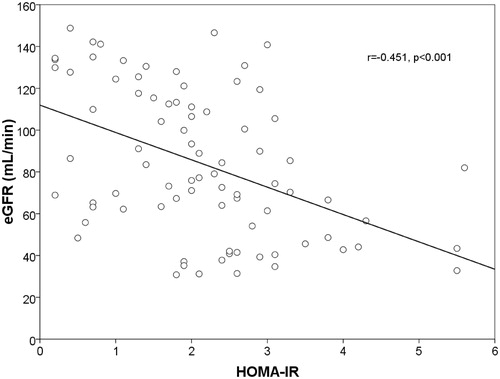
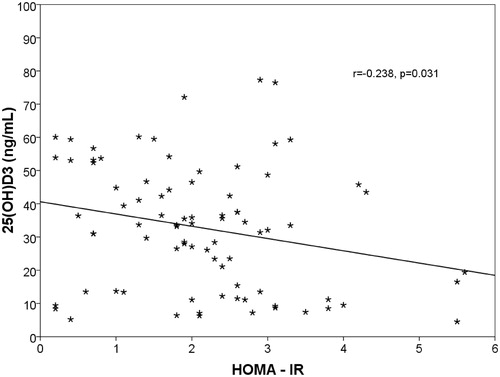
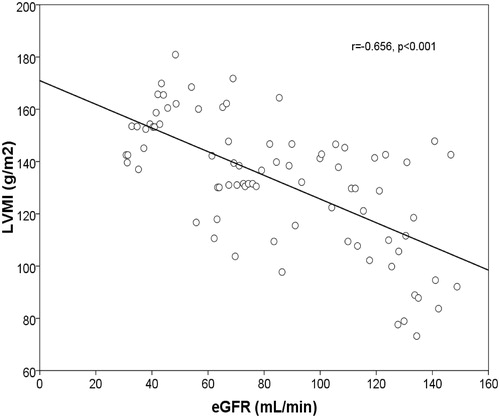
The insulin and intact PTH levels were increased in CKD patients, whereas the serum levels of 25(OH)D were significantly reduced (p = 0.044, p = 0.012, p = 0.038). A positive correlation was found between LVMI and IR (r = 0.669, p = 0.001) (). The increase of LVMI and IR were negatively correlated with eGFR (r = −656, p < 0.001) ( and ) and hemoglobin (r = −403, p = 0.036). In addition, a negative correlation was found between IR and Vit D levels (r = −0.238, p = 0.031) ().
Figure 1. Linear regression plot. LVMI (g/m2) vs. HOMA-IR. Positive correlation was found between the two parameters (r = 0.669, p < 0.001).
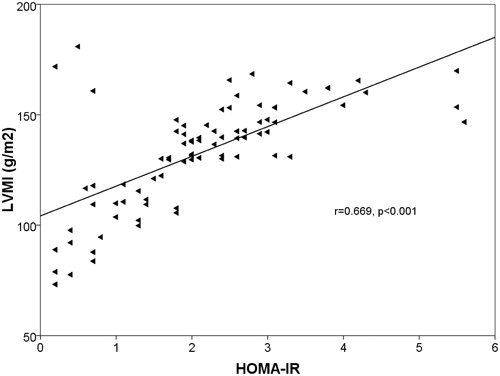
Figure 2. Linear regression plot. LVMI (g/m2) against eGFR(mL/min). Negative correlation was found between the two parameters (r = −0656, p < 0.001).
Discussion
The excess CV mortality in patients with CKD is not explained by traditional risk factors, such as hypertension and hypercholesterolemia. Identifying and treating risk factors for early CKD may be the best approach to prevent and delay adverse outcome.Citation2,Citation3
This study shows a relationship between IR, vit D deficiency, and vascular disease in nondiabetic, predialysis CKD patients. This relationship is independent of conventional CV risk factors. IR, as measured by HOMA-IR, and vit D deficiency were found to be independent predictors of LVH and atherosclerotic disease (cIMT) in the multivariate regression analysis after adjusting for the traditional risk factors.
Vitamin D deficiency is a highly prevalent condition, present in approximately 30% to 50% of the general population. A growing body of data suggests that low 25(OH)D levels may adversely affect CV health. VDRs are present on a large variety of cell types, including myocytes, cardiomyocytes, pancreatic beta-cells, vascular endothelial cells, neurons, immune cells, and osteoblasts. Directly or indirectly, 25(OH)2D regulates more than 200 genes, including those involved in renin production in the kidney, insulin production in the pancreas, the release of cytokines from lymphocytes, cathelicidin production in macrophages, and the growth and proliferation of both vascular smooth muscle cells and cardiomyocytes, and can predispose to hypertension, LVH, and CV disease.Citation6,Citation34 Vitamin D deficiency reduces intestinal calcium absorption by more than 50% and determines an increase in the levels of PTH.Citation35
An increased PTH level is associated with left ventricular and vascular medial smooth muscle hypertrophy. Vitamin D deficiency and/or increased PTH also predispose to calcification of the heart valves, mitral annulus, and myocardium, especially in patients with moderate or severe CKD.Citation36
Serum levels of 25(OH)D are inversely related to hypertension, carotid atherosclerosis, myocardial infarction, congestive heart failure, stroke, microalbuminuria, and kidney dysfunction; however, neither the role of vit D deficiency in the development of CV disease nor the practical recommendation for its supplementation to prevent CV disease has been established.Citation37
Recent observational studies of patients with CKD and hyperparathyroidism found that oral administration of activated vit D was associated with significantly improved survival.Citation38 A placebo-controlled study of 30 predialysis renal failure patients with secondary hyperparathyroidism showed that treatment with calcitriol improved left ventricular diastolic function.Citation39 Nutritional supplementation with vit D has been shown to reverse LVH in adult CKD patients receiving hemodialysis and in some animal models.Citation40,Citation41
IR is prevalent in CKD patients, even when the glomerular filtration rate is within the normal range, and plays a role in declining renal function. The etiology of IR is multifactorial and associated with a complex network that includes chronic inflammation, oxidative stress, vit D deficiency, anemia, and malnutrition.Citation42
The observation that IR is independent of obesity (BMI) and glucose intolerance in CKD implies that greater surveillance is warranted because IR is a modifiable risk factor. Lifestyle interventions, such as diet and exercise, improve insulin sensitivity and endothelial function. Pharmacological agents, such as ACEIs and ARBs, statins, metformin, PPARγ agonists, and, more recently, acetyl-l-carnitine, have also been shown to be beneficial. However, their effect on improving tissue responsiveness to insulin in CKD has yet to be proven. Nevertheless, IR is an independent risk factor for CV mortality in nondiabetic patients with CKD, as shown by Shinohara and colleaguesCitation39; therefore, the identification of patients with IR may help stratify those with increased CV risk.Citation7,Citation21
IR may develop in the presence of inflammation. Also, chronic inflammation has been shown to be an independent predictor of CV mortality. Therefore, there is a possibility that the observed association between increased HOMA-IR and CV mortality was mediated by chronic inflammation. However, this study showed that the effect of HOMA-IR is independent of C-reactive protein. These data could indicate that IR and chronic inflammation independently affect CV mortality in the CKD population or in more advanced disease stages.Citation43
Limitations of the study
Our study has several limitations that need consideration. First, only serum 25(OH)D, but not 1,25-dihydroxyvitamin D, was measured because serum 25(OH)D is currently used by the K/DOQI to define vit D deficiency. Second, our study had a relatively small, selective cohort of nondiabetic, predialysis CKD patients and thus needs to be repeated in a larger population. Third, our study is based on associations with surrogate end points; the generated hypothesis thus needs further prospective follow-up studies with hard end points to show causality.
Furthermore, a significant proportion of CKD patients were on antihypertensives and statins; therefore, the potential impact of hypertension, hypercholesterolemia, and their treatment on IMT may have confounded our results.
Conclusion
In conclusion, this study showed that IR is presented by early CKD patients without clinical diabetes; therefore, the detection and treatment of IR should be considered even in nondiabetic patients in the early stages of CKD.
IR had a significant correlation with LVH and may be an important risk factor for the development of CV disease. Because IR is a modifiable risk factor, it may be an important therapeutic target for reduction of CV mortality in these patients.Citation7
In our study, vit D deficiency was positively associated with LVH and atherosclerotic disease in the early stages of CKD, independent of age, BMI, diabetic status, and smoking. Vitamin D supplementation is simple, safe, and inexpensive; thus, it is recommended in these patients.Citation6,Citation44 Further studies are warranted to establish the causal relationship between IR, vit D deficiency, and CKD.
Practical application
Monitoring of serum 25(OH)D levels and IR, as well as appropriate treatment, is indicated in the CKD population as a potential strategy for preventing excess CV mortality.Citation45
Declaration of interest
The authors report no conflicts of interest. The manuscript has been seen and approved by all authors. This study was not funded. The manuscript is not under consideration for publication elsewhere.
References
- Kasiske BL, Chavers B, Foley R, et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2) suppl 1:S1–S266
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5):S112–S119
- Muntner P, He J, Hamm L, Loria C, Whelton PK. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002;13(3):745–753
- Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Hypertension. 2003;42:1050–1065
- Kobayashi S, Maesato K, Moriya H, Ohtake T, Ikeda T. Insulin resistance in patients with chronic kidney disease. Am J Kidney Dis. 2005;45(2):275–280
- Lee JH. Vitamin D deficiency: an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52(24):1949–1956
- Banerjee D. Insulin resistance, inflammation, and vascular disease in nondiabetic predialysis chronic kidney disease patients. Clin Cardiol. 2011;34(6):360–365
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281
- Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25 hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–777
- Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165
- Herzog CA, Asinger RW, Berger AK, et al. Cardiovascular disease in chronic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80(6):572–586
- Li YC. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem. 2003;88:327–331
- Xiang W, Kong J, Chen S, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125–E132
- Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol. 2007;103:521–524
- Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology. 2008;149(2):558–564
- Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(suppl):S1–S201
- Dzurik R, Spustova V, Janekova K. The prevalence of insulin resistance in kidney disease patients before the development of renal failure. Nephron. 1995;69:281–285
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607
- Howard G, O’Leary DH, Zaccaro D, et al. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation. 1996;93:1809–1817
- Agewall S, Fagerberg B, Attvall S, Wendelhag I, Urbanavicius V, Wikstrand J. Carotid artery wall intima-media thickness is associated with insulin-mediated glucose disposal in men at high and low coronary risk. Stroke. 1995;26:956–960
- Ikizler TA. Nutrition, inflammation and chronic kidney disease. Curr Opin Nephrol Hy. 2008;17(2):162–167
- Liao MT, Sung CC. Insulin resistance in patients with chronic kidney disease. J Biomed Biotechnol. 2012;2012:691369
- Becker B, Kronenberg F, Kielstein JT, et al. Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: the mild and moderate kidney disease study. J Am Soc Nephrol. 2005;16:1091–1098
- Levey AS. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766–72
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419
- Shoji T, Emoto M, Nishizawa Y. HOMA index to assess insulin resistance in renal failure patients. Nephron. 2001;89:348–349
- Ho CY, Solomon SD. A clinician's guide to tissue Doppler imaging. Circulation. 2006;113(10):e396–e398
- Lang RM, Bierig M, Devereux RB, et al. Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463
- Otterstad JE, Froeland G, St John SM, Holme I. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J. 1997;18:507–513
- Garcia MJ, Thomas JD, Klein AL. New Doppler echocardiographic applications for the study of diastolic function. J Am Coll Cardiol. 1998;32:865–875
- Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367
- Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92:39–48
- Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013
- Lee GH, Benner D, Regidor DL, Kalantar-Zadeh K. Impact of kidney bone disease and its management on survival of patients on dialysis. J Ren Nutr. 2007;17:38–44
- Mitsuhashi T, Morris RC Jr, Ives HE. 1,25-Dihydroxyvitamin D3 modulates growth of vascular smooth muscle cells. J Clin Invest. 1991;87:1889–1895
- Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med. 2008;168:397–403
- Singh NP, Sahni V, Garg D, Nair M. Effect of pharmacological suppression of secondary hyperparathyroidism on cardiovascular hemodynamics in predialysis CKD patients: a preliminary observation. Hemodial Int. 2007;11:417–423
- Matias PJ, Jorge C, Ferreira C, et al. Cholecalciferol supplementation in hemodialysis patients: effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol. 2010;5:905–911
- Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2-vitamin D3 and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest. 1996;97:1577–1588
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–1801
- Shinohara K, Shoji T. Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. JASN. 2002;13(7):1894–1900
- Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759
- Thadhani R. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307:674–684
- Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511