Abstract
Renal dysfunction is associated with mortality in patients after ischemic stroke. Cystatin C is a potentially superior marker of renal function compared to creatinine and estimated glomerular filtration rate (GFR). In our observational cohort study, 390 Caucasian patients suffered from acute ischemic stroke (mean age 70.9 years; 183 women and 207 men) were included and prospectively followed up to maximal 56 months. Serum creatinine and cystatin C were measured at admission to the hospital; GFR was estimated according to CKD-EPI creatinine and CKD-EPI creatinine/cystatin equations. According to values of serum creatinine, estimated GFR and serum cystatin C patients were divided into quintiles. In the follow-up period, 191 (49%) patients died. For serum cystatin C and estimated GFR based on creatinine and cystatin C, the mortality and the hazard ratios for long-term mortality increased from the first to the fifth quintile nearly linearly. The associations of serum creatinine and estimated GFR categories based on creatinine with long-term mortality were J-shaped. As compared with lowest quintile of serum cystatin C, the fifth quintile was associated with long-term mortality significantly also after multivariate adjustment (age, gender, initial stroke severity, known risk factors for stroke mortality). In contrast, in adjusted analysis serum creatinine and estimated GFR (CKD-EPI creatinine and CKD-EPI creatinine/cystatin) were not associated with long-term mortality. In summary, serum cystatin C was independently and better associated with the risk of long-term mortality in patients suffering from ischemic stroke than were creatinine and estimated GFR using both CKD-EPI equations.
Introduction
Coronary heart disease and stroke are the main forms of the cardiovascular disease (CVD) which is the leading cause of death in Europe, United States, and in many other developed countries.Citation1,Citation2 Renal dysfunction carries a substantial risk for cardiovascular morbidity and mortality, the risk increases with a decline in kidney function.Citation3–5 Patients with chronic kidney disease (CKD) and end-stage renal failure have advanced atherosclerosis of cerebral vasculature and have a higher risk for ischemic stroke.Citation6–8 In several studies, higher prevalence of renal dysfunction in patients with ischemic stroke was reported.Citation9,Citation10 Furthermore, renal dysfunction was also an independent predictor of mortality in patients with stroke.Citation9,Citation11–13 Patients with renal dysfunction are also at greater risk of future ischemic stroke.Citation14
Estimation of the glomerular filtration rate (GFR) is essential for the evaluation of patients with kidney disease. Over the last years, serum creatinine and creatinine-based formulas were the most commonly used markers to estimate GFR in clinical practice as in most studies.Citation15,Citation16 One such equation, the abbreviated Modification of Diet in Renal Disease (MDRD) Study equation, was widely accepted and reported by most clinical laboratories.Citation17 Unfortunately, serum creatinine and estimated GFR based on creatinine are influenced by several variables (sex, age, race, muscle mass, weight, drugs, etc.) and formulas are limited by lack of validation in the full range of GFR to which they are applied.Citation16,Citation18 Particularly, mild and moderate declines in renal function are inaccurately displayed by these markers and as a consequence a number of patients who are already at a higher risk for cardiovascular events and mortality remain undetected.Citation16,Citation18 To minimize limitations, in 2009 new and more accurate the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI creatinine equation) was developed and is now replacing MDRD equation.Citation19 In the last years serum cystatin C, low-molecular-weight protease inhibitor, that is freely filtered across the glomerular membrane and then reabsorbed and metabolized in the proximal tubule, was proposed as a new endogenous marker of GFR. Serum cystatin C is a more sensitive parameter for renal function assessment compared to serum creatinine or estimated GFR based on creatinine.Citation20 Serum cystatin C does not depend on muscle mass, sex or age, nor is affected by inflammation, fever, and/or outside agents.Citation21,Citation22 There are contradictory data considering the possible influence of malignant diseases on the serum concentration of cystatin C; however, most authors share the opinion that malignant processes do not influence the serum cystatin C concentration.Citation21,Citation23
Serum cystatin C has limitations as a marker of kidney function in certain disease states (thyroid disorders, glucocorticoid therapy).Citation24,Citation25 It was shown that serum cystatin C is superior for prediction of mortality and cardiovascular events among elderly and among elderly diabetic patients compared to serum creatinine or estimated GFR based on creatinine.Citation26,Citation27 In the past years, also equations based on both creatinine and cystatin C were developed, widely accepted is the CKD-EPI creatinine/cystatin equation.Citation28
It was also shown that serum cystatin C is a strong predictor of death in patients suffering from stroke, but there are no data about predicting long-term mortality in these patients comparing serum cystatin C and estimated GFR based on creatinine or creatinine and cystatin C.Citation29
The aim of our study was to compare the associations of serum creatinine, estimated GFR (CKD-EPI creatinine and CKD-EPI creatinine/cystatin equations), serum cystatin C and long-term mortality risk in patients suffering from ischemic stroke.
Patients and methods
In our observational cohort study, 390 Caucasian patients suffered from acute ischemic stroke and hospitalized at our department in one year period were included. Patients were prospectively followed up from the day of the admission to the hospital until their death or maximal 56 months (from 1 to 1673 days). Acute ischemic stroke was defined according to World Health Organization criteria.Citation30 The stroke was diagnosed in patient with appropriate clinical event and was confirmed with computer tomography (CT). The patients with transitory ischemic attack (TIA) were excluded from the study. A study neurologist reviewed all cases. At the admission, a quantitative measurement of neurologic deficit was performed according to the National Institutes of Health Stroke Scale (NIHSS1).Citation31 No included patient had comorbid malignancy, clinically thyroid disease, and no patient was on glucocorticoid therapy. No patient had clinically important muscle disease which can affect serum creatinine level and estimated GFR based on creatinine.
Serum creatinine and serum cystatin C were measured at admission to the hospital. Serum creatinine was measured by using the kinetic method according to Jaffé without deproteinization (Roche Diagnostics). This is a compensated method based on manufacturer instructions and was described previously.Citation32 Serum cystatin C was measured by the particle-enhanced immunonephelometric method (Dade Behring). GFR was estimated according to CKD-EPI creatinine and CKD-EPI creatinine/cystatin equation.Citation15,Citation19,Citation28 According to the values of serum creatinine, estimated GFR (CKD-EPI creatinine and CKD-EPI creatinine/cystatin equations) and serum cystatin C patients were divided into quintiles. For estimated GFR, the fifth quintile included patients with the lowest values (lower values are associated with worse renal function).
Data of previous diseases (hypertension and/or diabetes), heredity, and smoking habits were collected by questionnaire from patients and/or their relatives (aphasic patients, etc.). Diabetes mellitus was defined if the patient was already treated for diabetes mellitus (information obtained by a questionnaire from patients and/or their relatives) or fasting glucose value during hospital treatment was higher than 7 mmol/L.Citation33 The arterial hypertension was defined as present if the patient was already treated for hypertension (information obtained by a questionnaire from patients and/or their relatives) or the average measure blood pressure was ≥140 mmHg systolic or ≥90 mmHg diastolic based on three different measurements during hospitalization.Citation34 According to smoking habits, patients were divided in two subgroups: smokers (present) and non-smokers. According to patient’s weight and height, the body mass index (BMI) was calculated. In the first 24 hours, serum cholesterol (total, LDL, and HDL cholesterol), triglycerides, and high-sensitive C-reactive protein (hsCRP) were measured.
The ECG was recorded and the strips were reviewed by a study cardiologist; atrial fibrillation was documented.
The informed consent was obtained from each patient. The study protocol confirms to the ethical guidelines of the 1975 Declaration of Helsinki. The study was approved by National Medical Ethic Committee of the Republic of Slovenia.
Statistical methods
SPSS statistical software (version 19.0.1; IBM Corporation, Armonk, NY) was used for the analyses. Arithmetic mean values and standard deviation were calculated. Mortality and hazard ratio for each quintile of serum creatinine, estimated GFR (CKD-EPI creatinine and CKD-EPI creatinine/cystatin equations), and cystatin C were calculated. Cox regression analyses were used to evaluate the association of each measure of renal function (serum creatinine, estimated GFR, and cystatin C) with mortality. In adjusted model, we included variables which are known risk factors for stroke mortality: age, gender, initial stroke severity (NIHSS), heredity for stroke, hypertension, smoking, diabetes, atrial fibrillation, total cholesterol, triglycerides, hsCRP, and BMI.
Results
The mean age of included patients was 70.9 ± 11.64 years (ranged from 36 to 96 years), 183 were women (46.9%) and 207 (53.1%) were men. Age increased significantly from the first to fifth quintile of serum creatinine, estimated GFR (CKD-EPI creatinine and CKD-EPI creatinine/cystatin equations), and serum cystatin C. Other baseline data of our patients are presented in .
Table 1. Some basic data of our patients included in the study.
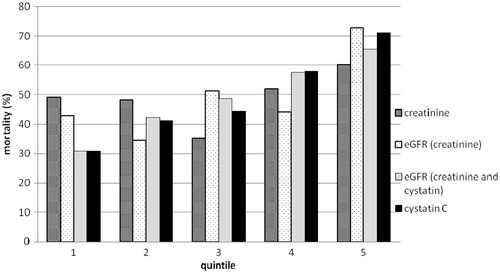
In the follow-up period, 191 (49%) patients died. The mortality according to quintiles of serum creatinine, estimated GFR (CKD-EPI creatinine and CKD-EPI creatinine/cystatin equations), and serum cystatin C is presented in . The association of serum creatinine categories with mortality was J-shaped; the association of estimated GFR categories based on creatinine (CKD-EPI creatinine equation) with mortality also appeared to be J-shaped. The serum cystatin C categories were nearly linearly, in a dose–response manner, associated with mortality. The association of estimated GFR categories based on creatinine and cystatin (CKD-EPI creatinine/cystatin equation) were also linearly associated with mortality.
Figure 1. Long-term mortality in patients after ischemic stroke in each quintile of serum creatinine, estimated GFR according to CKD-EPI creatinine equation, estimated GFR according to CKD-EPI creatinine and cystatin C equation, and cystatin C.
The hazard ratios for mortality according to quintiles of serum creatinine, estimated GFR (CKD-EPI creatinine and CKD-EPI creatinine/cystatin equations), and cystatin C are presented in . The association of hazard ratios for serum creatinine was J-shaped. For the estimated GFR based on creatinine (CKD-EPI creatinine equation) only, the fifth quintile hazard ratio for mortality was significantly higher compared to the first quintile. For the serum cystatin C, hazard ratios increased from the first to the fifth quintile nearly linearly. The hazard ratio for mortality was 2.126 (CI: 1.308–3.456; p = 0.002) in the fourth quintile and 3.429 (CI: 2.131–5.519; p = 0.0001) in the fifth quintile compared to the first quintile.
Table 2. Mortality and hazard ratios for death according to measures of renal function.
For the estimated GFR based on creatinine and cystatin C (CKD-EPI creatinine/cystatin equation), hazard ratios also increased from the first to the fifth quintile nearly linearly. The hazard ratio for mortality was significantly higher in the third (1.742, CI: 1.045–2.905; p = 0.03), in the fourth (2.211, CI: 1.347–3.630; p = 0.002), and in the fifth (2.830, C: 1.740–4.601; p = 0.0001) quintile compared to the first quintile.
As compared with the lowest quintile of serum cystatin C, the fifth quintile was associated with mortality significantly after multivariate adjustment for age, gender, initial severity of stroke (NIHSS), heredity for stroke, hypertension, smoking, diabetes, atrial fibrillation, cholesterol, triglycerides, hsCRP, and BMI (hazard ratio 1.938, CI: 1.124–3.344; p = 0.017) (). In contrast, in adjusted analysis, serum creatinine and estimated GFR based on creatinine or on creatinine and cystatin C (CKD-EPI creatinine and CKD-EPI creatinine/cystatin equations) were not associated with mortality ().
Discussion
In our study, the mortality in the follow-up period (up to 56 months) in patients suffering from ischemic stroke was approximately 50%. Serum cystatin C was independent and strong predictor of risk of long-term mortality in patients after ischemic stroke. The mortality and hazard ratios increased from the first to the fifth quintile of serum cystatin C nearly linearly. The fifth quintile was associated with long-term mortality significantly also after multivariate adjustment including age, initial stroke severity (NIHSS), and most risk factors for mortality in patients suffering from ischemic stroke. The cystatin C values in the fifth quintile are above 1.53 mg/L; according to simple cystatin C formula, cystatin C values >1.53 mg/L correspond to estimated GFR < 65 mL/min/1.73 m2. Simple cystatin C formula is a simple and reliable marker of estimated GFR in different patients populations (CKD patients, elderly, diabetic patients, etc.).Citation18,Citation35,Citation36
The associations of serum creatinine and estimated GFR categories based on creatinine (CKD-EPI creatinine equation) with long-term mortality were more or less J-shaped; serum creatinine and estimated GFR based on creatinine were not associated with long-term mortality after multivariate adjustment. Probably patients with mild-to-moderate declines in renal function were inaccurately displayed by serum creatinine and estimated GFR based on creatinine despite using more accurate CKD-EPI equation. Previously published meta-analysis including data from 1.1 million adults, from 25 general population cohorts, 7 high-risk cohorts, and 13 CKD cohorts, where participants in each category of estimated GFR by the MDRD Study equation were reclassified by the CKD-EPI equation, showed that CKD-EPI equation not only classified fewer individuals as having CKD but also more accurately categorized the risk for mortality than did the MDRD Study equation.Citation37 Despite using CKD-EPI equation in our study, the consequence was that a number of patients who were already at a higher risk for mortality remained undetected.
If GFR was estimated based on creatinine and cystatin C (CKD-EPI creatinine/cystatin equations), the mortality and the hazard ratios increased linearly from the first to the fifth quintile but after multivariate adjustment estimated GFR categories based on creatinine and cystatin C were not independent predictors of risk of long-term mortality. In the study by Astor et al.,Citation38 6942 adult participants in the Third National Health and Nutritional Examination Survey (NHANES III) were included. The association of estimated GFR based on creatinine and cystatin C compared with mortality was weaker compared to the association of cystatin C with mortality in general population.Citation38 The results of our study are also consistent with studies showing that cystatin C is a better estimate of GFR than serum creatinine and estimated GFR based on creatinine, particularly in the “normal” range of kidney function.Citation18,Citation39,Citation40 Previously it has been shown that serum cystatin C is associated better with mortality than creatinine and estimated GFR based on creatinine in eldery,Citation26 in patients with coronary heart disease,Citation41 heart failure,Citation42 and also in patients with peripheral arterial disease.Citation43 Serum cystatin C was a strong and independent predictor of mortality also in patients with normal to moderately impaired renal function.Citation42,Citation44,Citation45 In these studies, GFR was estimated by using less accurate Cockcroft–Gault or MDRD Study equations. It is interesting that after multivariate analysis in the study by Shlipak et al. in the elderly, the first and the second quintiles of serum cystatin C had similar long-term mortality rate, the third and the fourth quintiles were associated with moderately increased risk, and fifth quintile was clearly associated with increased risk of long-term mortality.Citation26 Serum creatinine and estimated GFR based on creatinine appeared to have J-shaped association with the risk of death in this study.Citation26 This J-shaped association with mortality persisted in the multivariate analysis.Citation26 Astor et al. compared cystatin C and GFR estimated by using CKD EPI equation as predictors of mortality in participants from The Atherosclerosis Risk in Communities (ARIC) study.Citation46 Serum cystatin was associated more strongly with mortality than estimated GFR based on creatinine; for estimated GFR only the lowest category (GFR < 60 mL/min/1.73 m2) was associated significantly with a greater incidence of mortality.Citation46 This study showed that although estimated GFR was associated with increased risk, the risk was lower than that observed for cystatin C.Citation46 Possible reasons for the stronger association between serum cystatin C and mortality compared to creatinine and estimated GFR are not completely clear, but are likely due, at least in part, to non-GFR determinants of serum cystatin C.Citation47
In previous studies, it was documented that renal dysfunction is independent predictor of short- and long-term mortality in patients with stroke.Citation9–14 In most studies, creatinine or estimated GFR based on creatinine were used to define renal dysfunction. The predictive value of cystatin C has not been fully established in patients suffering from ischemic stroke. There is only study by Ni et al. presenting the role of serum cystatin C in predicting long-term mortality in patients suffering from ischemic stroke.Citation29 In this study, 293 stroke patients (199 cases of cerebral infarction) and 894 controls were included.Citation29 Controls were selected from inpatients with minor illnesses free of neurological diseases.Citation29 Higher serum cystatin C levels were found in patients than in controls and higher serum cystatin C levels were directly associated with a higher risk of further ischemic and hemorrhagic stroke.Citation29 Patients were followed up for 5 years and the hazard ratio for stroke increased stepwise from the first to the fifth quintile of serum cystatin.Citation29 Serum cystatin C was a strong predictor of the mortality in this study.Citation29 When plasma serum creatinine was evaluated, only the highest creatinine subgroup had significantly increased risk for cerebral infarction.Citation29 Renal function (GFR) was not estimated using equations in this study.Citation29 It is interesting that in study by Seliger et al. serum cystatin was associated with subclinical brain infarcts and there was a strong and linear association with subclinical brain infarcts for 1/cystatin C but not for 1/creatinine, for which a quadratic U-shaped association was suggested.Citation48 In study by Xiao et al., patients with higher serum cystatin C levels had larger cerebral infarct size.Citation49
There are some limitations of our analysis. First, only Caucasian patients were included in our study, so results should be confirmed in other populations. Second, despite serum creatinine and cystatin C were withdrawn at admission, in most patients within maximal few hours after stroke, minimal acute deterioration of renal function cannot be completely excluded. For important deterioration of renal function with impact on results more time is needed. We did not use a direct method of measuring GFR as a reference for cystatin C and estimated GFR so we cannot exclude the possibility that cystatin C affects prognosis by mechanisms unrelated to renal factors.
In summary, serum cystatin C was better and independently associated with the risk of long-term mortality in patients suffering from ischemic stroke. In contrast, neither serum creatinine nor estimated GFR based on creatinine or based on creatinine and cystatin C using CKD-EPI equations were significant predictors of long-term mortality in these patients. Further studies are needed to find out if this novel marker of renal function can improve our decisions in patient with ischemic stroke.
Declaration of interest
The authors report no conflicts of interest.
This work was partly supported by Slovenian Research Agency (ARRS); project Chronic Renal Failure—New Risk Factor for Stroke (L3-9376).
References
- Bhatnagar P, Scarborough P, Smeeton NC, Allender S. The incidence of all stroke and stroke subtype in the United Kingdom, 1985 to 2008: a systematic review. BMC Public Health. 2010;10:539
- Cooper R, Cutler J, Desvigne-Nickens P, et al. Trends and disparities in coronary heart diseases, stroke, and other cardiovascular disease in the United States: findings of the National Conference on cardiovascular Disease Prevention. Circulation. 2000;102(25):3137–3147
- Manjunath G, Tighiouart H, Coresh J, et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int. 2003;63(3):1121–1129
- Garg AX, Clark WF, Haynes RB, et al. Moderate renal insufficiency and the risk of cardiovascular mortality: results from the NHANES I. Kidney Int. 2002;61(4):1486–1494
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305
- Kawagishi T, Nishizawa Y, Konishi T, et al. High-resolution B-mode ultrasonography in evaluation of atherosclerosis in uremia. Kidney Int. 1995;48(3):820–826
- Hojs R. Carotid intima-media thickness and plaques in hemodialysis patients. Artif Organs. 2000;24(9):691695
- Seliger SL, Gillen DL, Longstreth WT Jr, Kestenbaum B, Stehman-Breen CO. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003;64(2):603–609
- Tsagalis G, Akrivos T, Alevizaki M, et al. Renal dysfunction in acute stroke: an independent predictor of long-term all combined vascular events and overall mortality. Nephrol Dial Transplant. 2009;24(1):194–200
- Yahalom G, Schwartz R, Schwammenthal Y, et al. Chronic kidney disease and clinical outcome in patients with acute stroke. Stroke. 2009;40(4):1296–1303
- Hojs Fabjan T, Hojs R, Tetickovic E, Pecovnik Balon B. Ischemic stroke — impact of renal dysfunction on in-hospital mortality. Eur J Neurol. 2007;14(12):1351–1356
- MacWalter RS, Wong SYS, Wong KYK. Does renal dysfunction predict mortality after acute stroke? A 7-year follow-up study. Stroke. 2002;33(6):130–135
- Ovbiagele B. Chronic kidney disease and risk of death during hospitalization for stroke. J Neurol Sci. 2011;301(1–2):46–50
- Lee M, Saver JL, Chang KH, Liao HW, Chang SC, Ovbigale B. Low glomerular filtration rate and risk of stroke: meta-analysis. BMJ. 2010;341:c4249
- National Kidney Fundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266
- Froissart M, Rossert J, Jacquot C, et al. Predictive performance of the modification of diet in renal disease and Cockcroft–Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16(3):763–773
- Miller WG. Reporting estimated GFR: a laboratory perspective. Am J Kidney Dis. 2008;52(4):645–648
- Hojs R, Bevc S, Ekart R, Gorenjak M, Puklavec L. Kidney function estimating equations in patients with chronic kidney disease. Int J Clin Pract. 2011;65(4):458–464
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). Ann Intern Med. 2009;150(9):604–612
- Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40(2):221–226
- Randers E, Erlandsen EJ. Serum cystatin C as an endogenous marker of renal function: a review. Clin Chem Lab Med. 1999;37(4):389–395
- Coll E, Botey A, Alvarez L, et al. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36(1):29–34
- Finney H, Williams AH, Price CP. Serum cystatin C in patients with myeloma. Clin Chim Acta. 2001;309(1):1–6
- Fricker M, Wiesli P, Brundle M, et al. Impact of thyroid dysfunction on serum cystatin C. Kidney Int. 2003;63(5):1944–1947
- Risch L, Herklotz R, Blumberg A, Huber AR. Effects of glucocorticoid immunosuppresion on serum cystatin C concentrations in renal transplant patients. Clin Chem. 2001;47(11):2055–2059
- Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352(20):2049–2060
- de Boer IH, Katz R, Cao JJ, et al. Cystatin C, albuminuria, and mortality among older adults with diabetes mellitus. Diabetes Care. 2009;32(10):1833–1838
- Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3418 individuals with CKD. Am J Kidney Dis. 2008;51(3):395–406
- Ni L, Lü J, Hou LB, et al. Cystatin C, associated with hemorrhagic and ischemic stroke, is a strong predictor of the risk of cardiovascular events and death in Chinese. Stroke. 2007;38(12):3287–3288
- Thorvaldsen P, Asplund K, Kuulasmaa K, et al. Stroke incidence, case fatality, and mortality in the WHO MONICA project. World Health Organisation Monitoring Trends and Determinants in Cardiovascular Disease. Stroke. 1995;26(3):361–367
- Muir KW, Weir CJ, Murray GD, Povey C, Lees KR. Comparison of neurological scales and scoring systems for acute stroke prognosis. Stroke. 1996;27(10):1817–1820
- Mazzachi BC, Peake MJ, Ehrhardt V. Reference range and method comparison studies for enzymatic and Jaffé creatinine assay in plasma and serum and early morning urine. Clin Lab. 2000;46(1–2):53–55
- Rydén L, Standl E, Bartnik M, et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J. 2007;28(1):88–136
- Mancia G, De Backer G, Dominiczak A, et al. Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25(6):1105–1187
- Bevc S, Hojs R, Ekart R, Gorenjak M, Puklavec L. Simple cystatin C formula compared to sophisticated CKD-EPI formulas for estimation of glomerular filtration rate in the elderly. Ther Apher Dial. 2011;15(3):261–268
- Bevc S, Hojs R, Ekart R, Završnik M, Gorenjak M, Puklavec L. Simple cystatin C formula for estimation of glomerular filtration rate in overweight patients with diabetes mellitus type 2 and chronic kidney disease. Exp Diabetes Res. 2012 . [Epub ahead of print]. doi:10.1155/2012/179849
- Matsushita K, Mahmodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD Study equation for estimated glomerular filtration risk. JAMA. 2012;307(18):1941–1951
- Astor BC, Levey AS, Stevens LA, Van Lente F, Selvin E, Coresh J. Method of glomerular filtration rate estimation affects prediction of mortality risk. J Am Soc Nephrol. 2009;20(10):2214–2222
- Sarnak MJ, Katz R, Stehman-Breen CO, et al. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med. 2005;142(7):497–505
- O'Riordan SE, Webb MC, Stowe HJ, et al. Cystatin C improves the detection of mild renal dysfunction in older patients. Ann Clin Biochem. 2003;40(Pt 6):648–655
- Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115(2):173–179
- Naruse H, Ishii J, Kawai T, et al. Cystatin C in acute heart failure without advanced renal impairment. Am J Med. 2009;122(6):566–573
- Urbonaviciene G, Shi GP, Urbonavicius S, Henneberg EW, Lindholt JS. Higher cystatin C level predicts long-term mortality in patients with peripheral arterial disease. Atherosclerosis. 2011;216(2):440–445
- Keller T, Messow CM, Lubos E, et al. Cystatin C and cardiovascular mortality in patients with coronary artery disease and normal or mildly reduced kidney function: results from the AtheroGene study. Eur Heart J. 2009;30(3):314–320
- Wu CK, Lin JW, Caffrey JL, Chang MH, Hwang JJ, Lin YS. Cystatin C and long-term mortality among subjects with normal creatinine-based estimated glomerular filtration rates: NHANES III (Third National Health and Nutrition Examination Survey). J Am Coll Cardiol. 2010;56(23):1930–1936
- Astor BC, Shafi T, Hoogeveen RC, et al. Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am J Kidney Dis. 2012;59(5):653–662
- Astor BC, Shaikh S, Chaundry M. Association of endogenous markers of kidney function with outcomes: more and less than glomerular filtration rate. Curr Opin Nephrol Hypertens. 2013;22(3):331–335
- Seliger SL, Longstreth WT Jr, Katz R, et al. Cystatin C and subclinical brain infarction. J Am Soc Nephrol. 2005;16(12):3721–3727
- Xiao D, Liu H, Zhang H, Luo Y. Impact of cystatin C levels on infarct size and hemorrhage volume in acute cerebral stroke. J Neurol. 2012;259(10):2053–2059
