Abstract
The association between monocyte chemoattractant protein-1 (MCP-1) -2518G/A gene polymorphism and the risk of nephropathy in type 2 diabetes mellitus (T2DM) remains controversial. A meta-analysis was conducted to assess the association of MCP-1 -2518G/A gene polymorphism with the risk of nephropathy in T2DM. Eight studies were included in our meta-analysis by searching electronic databases according to predefined criteria. No significant association between G allele, GG genotype, or AA genotype and the onset of nephropathy in T2DM was observed among Asians. GA genotype was significantly associated with nephropathy risk in T2DM among Asians (p = 0.024). MCP-1 -2518G/A gene polymorphism was not associated with nephropathy risk in T2DM among Chinese, Koreans, and Turks. For Indians, G allele and AA genotype were not associated with nephropathy risk in T2DM, GG genotype was associated with a lower risk of nephropathy in T2DM (p = 0.017), GA genotype was associated with the susceptibility of nephropathy in T2DM (p = 0.029). In conclusions, GA genotype might be a risk factor for the onset of nephropathy in T2DM among Asians, particularly Indians; GG genotype seems to be a protective factor against the susceptibility of nephropathy in T2DM among Indians.
Introduction
Diabetic nephropathy (DN) is a leading cause of chronic kidney disease, and once present will be likely to progress to end-stage renal disease.Citation1 DN complicates about 20–40% of type 2 diabetes mellitus (T2DM) cases, which suggests that certain factors predispose patients with T2DM to DN.Citation2 Currently, hypertension, poor glycemic regulation, and albuminuria are well-accepted risk factors for the onset of DN in T2DM.Citation3 However, the development of a number of DN cases remains unexplained.
Recently, tissue macrophage accumulation is thought to promote the development and progression of DN.Citation4 Macrophage infiltration is observed during the moderate stage of diffuse diabetic glomerulosclerosis in the renal biopsy specimen from diabetic cases.Citation5 Infiltrated macrophages release various inflammatory mediators that would lead to inflammation, interstitial fibrosis, and finally permanent kidney damage.Citation6 Monocyte chemoattractant protein-1 (MCP-1) is a strong stimulator of macrophage recruitment. Hence, MCP-1 is believed to be a pathogenic mediator in the development and progression of DN. Genetic variations in the gene encoding the MCP-1 might confer susceptibility to DN.Citation7 Several polymorphisms have been identified in the MCP-1 gene. -2518 A/G polymorphism in the distal regulatory region of MCP-1 is believed to regulate gene expression and influence plasma MCP-1 concentration.Citation8 In this sense, it is reasonable to postulate that MCP-1 -2518 A/G gene polymorphism is associated with the risk of nephropathy in T2DM.
The past few years have witnessed an increasing interest in testing this hypothesis among Asians. However, the results were in conflict among the reported studies.Citation6,Citation9–15 An improved understanding of this issue may have important clinical implications provided the possibility that the MCP-1 -2518 A/G gene polymorphism may be an indicator to predict the onset of nephropathy in T2DM. Meta-analysis is a good way to summarize the available evidence to provide more robust results than the individual study. Thus, we performed this meta-analysis to investigate the association between MCP-1 -2518 A/G gene polymorphism and nephropathy risk in T2DM with the aim of providing a much more reliable finding on the significance of the association.
Materials and methods
Search strategy
We attempted to search the published literature related to the association between MCP-1 -2518G/A gene polymorphism and the risk of nephropathy in T2DM using Pub Med, Embase, Cochrane, and China National Knowledge Infrastructure (CNKI) databases through May 2013. No restriction was imposed on search language. The used search terms were as follows: (1) type 2 diabetes mellitus, T2DM, nephropathy; and (2) monocyte chemoattractant protein-1, MCP-1, -2518G/A, gene polymorphism. Bibliographies of retrieved articles and reviews were also scrutinized. If the same data was included in more than one study, we chose the study with the most complete analysis.
Inclusion criteria
A case-control study
The outcome of interest was DN.
At least two comparison groups (DN group vs. T2DM group)
Exclusion criteria
Case reports, reviews, and editorials
Association of other genes with DN risk
Multiple publications of the same data
Investigation of the role of MCP-1 to diseases
Data extraction and synthesis
The following characteristics from each study were extracted: first author’s surname, year of publication, region of study population, number of cases and controls for MCP-1 -2518G/A genotype. Frequencies of G allele were calculated for case and control groups, from the corresponding genotype distribution. Two authors independently performed the data extraction and synthesis with any disagreements resolved by discussion.
Statistical analysis
STATA version 12.0 (Stata Corp, College Station, TX) was used to calculate the available data from each study. Odds ratio (OR) was used to measure the association between MCP-1 -2518G/A gene polymorphism and nephropathy risk in T2DM across studies. Heterogeneity of ORs among studies was tested by using the Q statistic (significance level at p < 0.10). The I2 statistic, a quantitative measure of inconsistency across studies, was also calculated. The pooled ORs were calculated using either a fixed-effects model or, in the presence of heterogeneity, a random-effects model. In addition, 95% confidence intervals (CIs) were also calculated. The OR was calculated by using four methods: method 1, allele comparison (G allele vs. A allele); method 2, comparing GG homozygous with the other two combinations (GG vs. GA + AA); method 3, comparing AA genotype with the other two combinations (AA vs. GA + GG); method 4, comparing GA genotype with the other two combinations (GA vs. GG + AA). A chi-square test using a web-based program was used to determine whether genotype distribution of the control groups reported conformed to Hardy–Weinberg equilibrium (HWE) (HWE; p < 0.05 was considered significant). Potential publication bias was assessed for overall Asians by Begg’s test and Egger’s test at the p < 0.05 level of significance. p < 0.05 was considered statistically significant, except where otherwise specified.
Results
Literature search
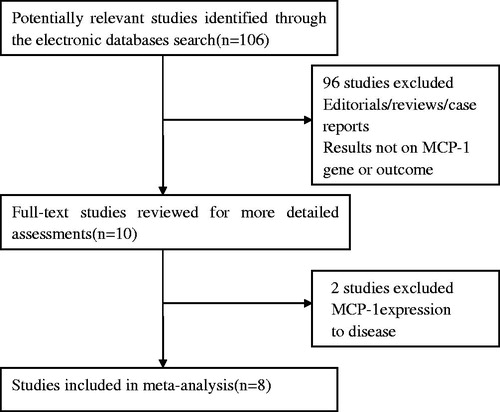
We firstly retrieved 106 references from the electronic databases. Of these, 98 publications were excluded according to the inclusion and exclusion criteria. Eight studiesCitation6,Citation9–15 were identified for the analysis of the association between MCP-1 -2518G/A gene polymorphism and nephropathy risk in T2DM ().
Study characteristics
A total of eight studies were recruited. Four studiesCitation10,Citation12,Citation13,Citation15 were conducted in China, twoCitation6,Citation11 in Korea, oneCitation14 in India, and oneCitation9 in Turkey (). A total of 908 cases and 806 controls were included (). The average frequency of the G allele was 47.9% in Chinese patients and 51.2% in controls (). For Koreans, the average frequency of G allele was 60.5% in the case group and 65.1% for controls (). For Indians, the average frequency of G allele was 33.8% in the case group and 33.1% for controls (). For Turks, the average frequency of G allele was 22.1% in the case group and 19.8% for controls (). The ratio of cases/controls for average frequency of G allele in Turks was higher compared with that in Indians, Chinese, and Koreans (Turks: cases/controls = 1.12; Indians: cases/controls = 1.02; Chinese: cases/controls = 0.94; Koreans: cases/controls = 0.93).
Table 1. Characteristics of studies evaluating the effects of MCP-1 -2518G/A gene polymorphism on DN risk.
Association between the MCP-1 -2518G/A gene polymorphism and nephropathy risk in T2DM
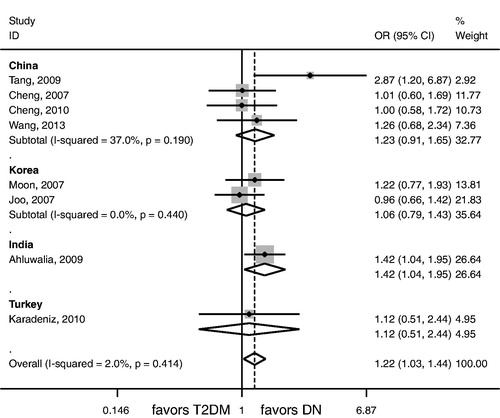
No significant association between G allele, GG genotype, or AA genotype and the risk of nephropathy in T2DM was observed among Asians (). GA genotype was significantly associated with nephropathy risk in T2DM among Asians (p = 0.024; ; ). MCP-1 -2518G/A gene polymorphism was not associated with nephropathy risk in T2DM among Chinese, Koreans, and Turks (). For Indians, G allele and AA genotype were not associated with nephropathy risk in T2DM (), GG genotype was associated with a lower risk of nephropathy in T2DM (p = 0.017; ), GA genotype was associated with the susceptibility of nephropathy in T2DM (p = 0.029; ; ).
Table 2. Meta-analysis of the association of MCP-1 -2518G/A gene polymorphism with DN risk.
Sensitivity analysis
The genotype distribution of the control group in one study was not in HWE. Hence, we included seven studies in the sensitivity analysis. For Asians, similar results regarding the association of G allele, GG genotype or AA genotype and nephropathy risk in T2DM were found in the sensitivity analysis compared with those in the non-sensitivity analysis (). Notably, an insignificant association was observed between GA genotype and the risk of nephropathy in T2DM (p = 0.084; ) which was a little different from that in the non-sensitivity analysis. For Chinese, similar results regarding the association of MCP-1 -2518G/A gene polymorphism and nephropathy risk in T2DM were found in the sensitivity analysis compared with those in the non-sensitivity analysis ().
Evaluation of publication bias
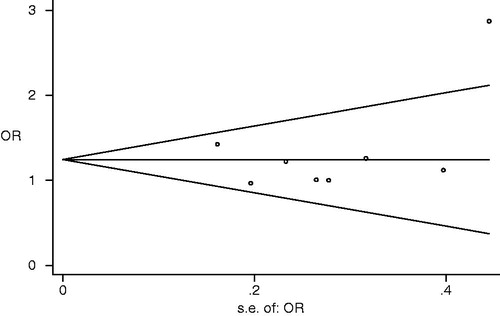
Visual inspection of funnel plot showed no significant asymmetry (). No evidence of significant publication bias for Asians was observed (G vs. A: Begg p = 0.621, Egger p = 0.852; GG vs. GA + AA: Begg p = 1, Egger p = 0.994; AA vs. GA + GG: Begg p = 0.37, Egger p = 0.296; GA vs. GG + AA: Begg p = 0.322, Egger p = 0.452).
Discussion
Increasing attention has been paid to the investigation of the etiology of nephropathy in T2DM. The confirmation of possible genetic origin of DN would give an insight to early diagnosis or intervention of DN. In our meta-analysis, we found that GA genotype was associated with the onset of nephropathy in T2DM among Asians and Indians, GG genotype was associated with a lower risk of nephropathy in T2DM among Indians. No evidence of publication bias for Asians was observed. Of note, the sensitivity analysis showed that an insignificant association between GA genotype and nephropathy risk in T2DM existed among Asians, which was a little from that in non-sensitivity analysis. Nevertheless, it still has important clinical significance that GA genotype may be a possible genetic marker for the onset of nephropathy in T2DM among Asians.
The regional differences might affect the association between MCP-1 -2518 A/G gene polymorphism and nephropathy risk in T2DM. In our investigation, the ratio of cases/controls for average frequency of G allele was 1.12, 1.02, 0.94, and 0.93 in Turks, Indians, Chinese, and Koreans, respectively. Although the ratio in Turks was the highest, no significant association between MCP-1 -2518 A/G gene polymorphism and nephropathy risk in T2DM was observed. This was mainly due to the fact that only one studyCitation9 in Turks was recruited, which made the results less robust. More studies should be performed in the future. In Indians, we found that GA genotype was associated with the nephropathy onset in T2DM, GG genotype was associated with a lower risk of nephropathy in T2DM. However, only one studyCitation14 in Indians was enrolled, more studies are needed to validate our results. For Chinese and Koreans, the results from the sensitivity analysis were similar to those from the non-sensitivity analysis, which indicated the results were relatively robust.
Several facts may explain the association between MCP-1 -2518 A/G gene polymorphism and nephropathy risk in T2DM. First, MCP-1 can directly stimulate macrophages to secrete increased levels of active and total TGF-β 1, which can promote production of extracellular matrix.Citation16 Meanwhile, TGF-β 1 can itself stimulate mesangial expression of MCP-1, which suggests a mechanism of self-amplifying cytokine-loop for promoting mesangial matrix accumulation.Citation17 In vitro studies also indicated that MCP-1 could promote renal fibrosis during diabetes.Citation18 MCP-1- deficient diabetic kidneys showed that a lack of MCP-1 reduces the accumulation of interstitial myofibroblasts and the deposition of glomerular and interstitial collagen type IV.Citation19 Second, kidney MCP-1 production plays a critical role in the development of diabetic renal inflammation, which leads to progression of DN.Citation20 Selective targeting of MCP-1 has been proven to be an effective therapy in suppressing animal models of DN.Citation21 Finally, the polymorphism of -2518G/A in the MCP-1 gene influences MCP-1 expression in response to inflammatory stimuli.Citation22 This polymorphism was associated with the production of MCP-1 by interleukin-1β-stimulated peripheral blood mononuclear cells.Citation8 All the above-mentioned data indicate a possible association of MCP-1 -2518G/A gene polymorphism with nephropathy risk in T2DM.
In the past, the association between MCP-1 -2518G/A gene polymorphism and renal diseases were investigated in a number of studies. Mori et al.Citation23 reported that AA genotype was an independent risk factor for the progression of renal disease in Japanese patients with IgA nephropathy. Malafronte et al.Citation24 reported that GG genotype was associated with the risk of lupus nephritis in Brazilians. Piotrowski et al.Citation25 reported that G variant was associated with renal manifestations of systemic lupus erythematous in Polishes. Kim et al.Citation26 reported that -2518G/A gene polymorphism was associated with nephritis in systemic lupus erythematous by modulating MCP-1 expression in Koreans. All these evidences support the possibility that MCP-1 -2518G/A gene polymorphism is associated with the development and progression of renal diseases.
Although no marked publication bias for Asians was observed, several limitations should be considered in our investigation. First, heterogeneities might be present, affecting the results of our meta-analysis, although a random-effects model had been performed. Second, the reporting bias might affect the results; further studies are needed to be performed in other ethnicities than Asians. Finally, the small sample size limited the statistical power. More studies should be performed in the future.
In conclusion, the results of our study suggest that GA genotype might be a risk factor for the onset of nephropathy in T2DM among Asians, particularly Indians; GG genotype seems to be a protective factor against the susceptibility of nephropathy in T2DM among Indians. However, larger number of studies in different regions and ethnicities are needed in the future.
Declaration of interest
There is no conflict of interest for all authors.
References
- Chen J, Muntner P, Hamm LL, et al. Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. J Am Soc Nephrol. 2003;14:469–477
- Olsen S, Mogensen CE. How often is NIDDM complicated with non-diabetic renal disease? An analysis of renal biopsies and the literature. Diabetologia. 1996;39:1638–1645
- Hovind P, Rossing P, Tarnow L, et al. Progression of diabetic nephropathy. Kidney Int. 2001;59:702–709
- Chow FY, Nikolic-Paterson DJ, Ma FY, et al. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia. 2007;50:471–480
- Furuta T, Saito T, Ootaka T, et al. The role of macrophages in diabetic glomerulosclerosis. Am J Kidney Dis. 1993;21:480–485
- Joo KW, Hwang YH, Kim JH, et al. MCP-1 and RANTES polymorphisms in Korean diabetic end-stage renal disease. J Korean Med Sci. 2007;22:611–615
- Maeda S. Do inflammatory cytokine genes confer susceptibility to diabetic nephropathy? Kidney Int. 2008;74:413–415
- Rovin BH, Lu L, Saxena R. A novel polymorphism in the MCP-1 gene regulatory region that influences MCP-1 expression. Biochem Biophys Res Commun. 1999;259:344–348
- Karadeniz M, Erdogan M, Cetinkalp S, et al. Monocyte chemoattractant protein-1 (MCP-1) 2518G/A gene polymorphism in Turkish type 2 diabetes patients with nephropathy. Endocrine. 2010;37:513–517
- Cheng J, Liu RH. Study on the correlation of the A2518G polymorphism of MCP-1 gene and diabetic nephropathy in type 2 diabetic subjects. Chin J Diabetes. 2007;15:87–88 (in Chinese)
- Moon JY, Jeong L, Lee S, et al. Association of polymorphisms in monocyte chemoattractant protein-1 promoter with diabetic kidney failure in Korean patients with type 2 diabetes mellitus. Korean Med Sci. 2007;22:810–814
- Tang HW, HU DL, LIU TJ, et al. A study on the association of MCP-1 gene polymorphism with type 2 diabetes and diabetic nephropathy in Zhuang minority population. Chin J Diabetes. 2009;17:430–436 (in Chinese)
- Wang Y, Chen JN, Jiang PP, et al. A correlation research between the monocyte chemoattractant protein-1 gene -2518G/A polymorphism and diabetic nephropathy. Chongqing Medicine. 2013;42:363–366 (in Chinese)
- Ahluwalia TS, Khullar M, Ahuja M, et al. Common variants of inflammatory cytokine genes are associated with risk of nephropathy in type 2 diabetes among Asian Indians. PLoS One. 2009;4:1–10
- Cheng J, Mo ZY, Feng YM, et al. Study on the correlation of the A2518G polymorphism of MCP-1 gene and diabetic nephropathy in type 2 diabetic subjects. China Clin Prac Med. 2010;4:74–75 (in Chinese)
- Wang SN, LaPage J, Hirschhberg R. Role of glomerular ultrafiltration of growth factors in progressive interstitial fibrosis in diabetic nephropathy. Kidney Int. 2000;57:1002–1014
- Cheng J, Diaz Encarnacion MM, Warner GM, et al. TGF-beta 1 stimulates monocyte chemoattractant protein-1 expression in mesangial cells through a phosphodiesterase isoenzyme 4-dependent process. Am J Physiol Cell Physiol. 2005;289:C959–C970
- Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294:697–701
- Chow FY, Nikolic-Paterson DJ, Ozols E, et al. Monocyte chemoattractant protein-1 promotes diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69:73–80
- Rivero A, Mora C, Muros M, et al. Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin Sci (Lond). 2009;116:479–492
- Kanamori H, Matsubara T, Mima A, et al. Inhibition of MCP-1/CCR2 pathway ameliorates the development of diabetic nephropathy. Biochem Biophys Res Commun. 2007;360:772–777
- Fenoglio C, Galimberti D, Lovati C, et al. MCP-1 in Alzheimer’s disease patients: A-2518G polymorphism and serum levels. Neurobiol Aging. 2004;25:1169–1173
- Mori H, Kaneko Y, Narita I, et al. Monocyte chemoattractant protein-1 A-2518G gene polymorphism and renal survival of Japanese patients with immunoglobulin A nephropathy. Clin Exp Nephrol. 2005;9:297–303
- Malafronte P, Vieira JM Jr, Pereira AC, et al. Association of the MCP-1 -2518 A/G polymorphism and no association of its receptor CCR2 -64 V/I polymorphism with lupus nephritis. J Rheumatol. 2010;37:776–782
- Piotrowski P, Lianeri M, Gasik R, et al. Monocyte chemoattractant protein-1 -2518 A/G single nucleotide polymorphism might be associated with renal disease and thrombocytopenia of SLE. Biomed Biotechnol. 2010;130265:1--6
- Kim HL, Lee DS, Yang SH, et al. The polymorphism of monocyte chemoattractant protein-1 is associated with the renal diseases of SLE. Am J Kidney Dis. 2002;40:1146–1152