Abstract
Waldenström’s macroglobulinemia (WM) is a rare lymphoid neoplasia, accounting for 2% of all hematological malignancies. Renal complications occur rather rarely compared to multiple myeloma. The most common renal manifestations are mild proteinuria and microhematuria. We describe a case of MW presenting with acute renal failure and NS. A 67-year-old man was referred to our hospital for sudden onset nephrotic syndrome. Electrophoresis revealed a monoclonal component in the gamma region, which was classified as an IgM k. During hospitalization, acute kidney injury developed, with creatinine up to 5 mg/dL, despite adequate hydration and alkalinization. A kidney biopsy was performed, showing minimal change disease (MCD) with interstitial and capsular lymphoid infiltrates of B-Lymphocytes CD20+. B-lymphocytes infiltration suggested the possibility of renal localization of lymphoproliferative disorder. So, bone marrow histology was performed, revealing lymphoplasmacytic lymphoma (WM). The patient was treated with bortezomib, desamethasone, and rituximab, with partial recovery of renal function (creatinine 1.5 mg/dL) and complete remission of proteinuria after 8-month follow-up. The remission of NS in our patient with rituximab seems to emphasize the pathogenetic role of B cells in MCD, although a coincident effect of immunosuppression on both the underlying renal disease and the hematologic disease cannot be excluded.
Introduction
Waldenström’s macroglobulinemia (WM), first described by J. Waldenström in 1944, is a rare lymphoid neoplasia, accounting for 2% of all hematological malignancies. The WHO defines WM as a lymphoplasmacytic lymphoma associated with a monoclonal immunoglobulin M (IgM) protein. Most WM patients have symptoms and signs related to tumoral infiltration (cytopenia and hepatosplenomegaly), monoclonal protein accumulation in the circulation (cryoglobulinemia and serum hyperviscosity syndrome), monoclonal protein accumulation in tissues (amyloidosis), or autoantibody production (neuropathy and hemolytic anemia).Citation1 Hepatomegaly occurs in 20%, splenomegaly in 15%, and lymphadenopathy in 15% of the patients. The most common presenting symptom is fatigue related to a normochromic normocytic anemia. The presence of ≥10% clonal lymphoplasmacytic cells in bone marrow confirms the diagnosis.Citation2
Renal complications occur rather rarely in WM compared to multiple myeloma. The most common renal manifestations are mild proteinuria and microhematuria. Nephrotic syndrome (NS) is rare. Bence-Jones proteinuria is present only in 10–15% of cases.Citation3 Only less than 3% of patients develop end-stage renal failure.Citation4
We describe a case of MW presenting with acute renal failure (ARF) and NS.
Case report
In January 2012, a 67-year-old man was referred to our hospital for sudden onset of edema, weight gain (10 kg during a month), and renal failure. Physical examination showed generalized edema, without hepatosplenomegaly or lymphadenopathy. Blood pressure was normal. Laboratory tests yielded the following values: hemoglobin 14.4 g/dL, white blood cell count 9390/mmCitation3 with 71% polymorphonuclear leukocytes and 18% lymphocytes, platelet count 428,000/mm3, urea 112 mg/dL, creatinine 1.8 mg/dL, creatinine clearance 38 mL/min, calcium 8.5 mg/dL, uric acid 5.5 mg/dL, LDH 191 U/L (n.v. <480), total protein 5.1 g/dL with albumin 1.4 g/dL, alpha1-globulin 6%, alpha2-globulin 18%, beta-globulin 9.6%, and gamma-globulin 39.5%. Blood glucose, hepatic tests, lipids, coagulation, HBV, and HCV markers were normal. Cryoglobulins were absent. Serum complement was normal. Urine sediment contained many granular casts and 10–15 dysmorphic erythrocytes. Proteinuria was 9.4 g/24 h. Electrophoresis revealed a monoclonal component in the gamma region, which was classified as an IgM k. Immunoglobulins and light chains were as follow: IgG 265 mg/dL (normal 681–1648), IgA 155 mg/dL (normal 87–474), IgM 2460 mg/dL (normal 48–312), kappa light chains 1296 mg/dL (normal 629–1350), lambda light chains 138 mg/dL (normal 313–723), kappa--lambda ratio 9.3. Bence-Jones proteinuria was negative.
Radiographic imaging of the bones and a total body computed tomography did not show, respectively, lytic lesions and organomegaly or lymphadenopathy. PET-CT scan was negative for any abnormal metabolic activity. Bone marrow aspiration was normal.
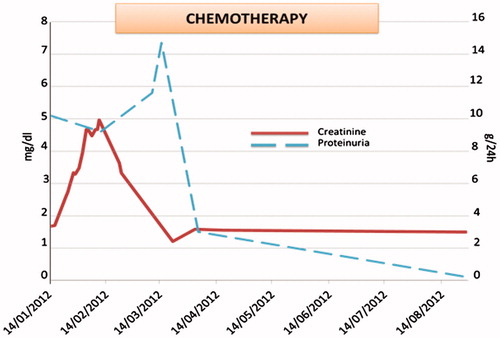
During hospitalization, acute kidney injury developed, with creatinine up to 5 mg/dL, despite adequate hydration and alkalinization. Renal US showed normal-sized kidneys with no signs of obstruction, and Doppler examination ruled out renal vein thrombosis.
A kidney biopsy was performed to determine the cause of proteinuria and decreased renal function.
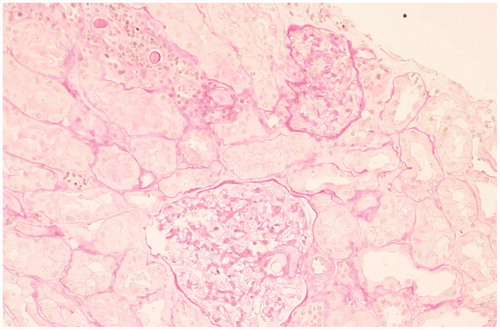
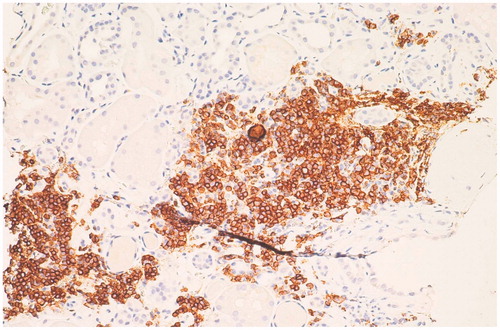
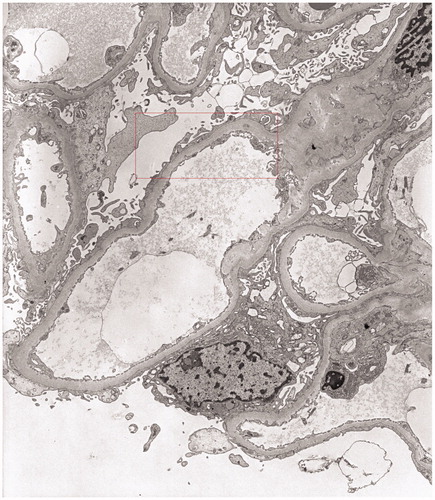
Renal biopsy showed minimal change disease (MCD) with small foci of parenchymal scleroatrophy (), interstitial and capsular lymphoid infiltrates of B-Lymphocytes CD20+ (). Immunofluorescence showed widespread granular deposition of IgM in mesangium. Stains for IgG, IgA, and kappa and lambda chains were negative. Congo red staining was negative. Electron microscopy did not reveal clear electron-dense deposits in the glomerular basement membrane but showed diffuse loss of podocyte foot processes ().
Figure 3. Electron microscopy: widespread foot process fusion in the absence of electron-dense deposits, UrPb (5000×).
B-lymphocytes infiltration suggested the possibility of renal localization of lymphoproliferative disorder. So, bone marrow histology was performed, showing micronodular infiltration of lymphoid cells with phenotype CD20+ CD5-(90%), CD 138+(10%), consistent with a diagnosis of lymphoplasmacytic lymphoma (WM).
The patient was treated with bortezomib, dexamethasone, and rituximab, with partial recovery of renal function (creatinine 1.5 mg/dL) and complete remission of proteinuria after 8-month follow-up ().
Discussion
Renal involvement in patients with WM is rare as compared with those of multiple myeloma, because hypercalcemia is uncommon and Bence-Jones proteinuria is usually small in amounts. In autopsy series of WM, only 3.8% to 7.4% of the patients developed renal failure. While proteinuria and microhematuria are not infrequent, incidence of NS is reported to be less than 7%. AL amyloidosis is commonly considered as the main cause of NS.Citation4 Moreover, there are sporadic reports of associated membranous nephropathy, MCD, crescentic glomerulonephritis, immune complex-mediated glomerulonephritis, cast nephropathy, and Fanconi’s syndrome. MCD is more frequently associated with Hodgkin’s lymphoma; to date, only three cases have been reported in literature in patients with MW.Citation5–7 However, direct infiltration of the kidney by atypical lymphoid cells remains the most common finding, occurring in 50–60% of the patients, together with large intracapillary aggregates of IgM (pseudothrombi).Citation8 Different patterns of renal damage can be present in the same patient. The largest series of renal symptoms and histologic lesions was described by Morel-Maronger et al. in 1970. Of 16 patients, only 5 underwent renal biopsy, while in the others renal histology was provided by autopsy. Three patients had NS, five patients presented with renal failure, and in two cases it was superimposed on NS. The most common histologic findings were intraglomerular thrombi, present in five cases; three patients had AL amyloidosis, one had only endomembranous deposits, and in seven cases no detectable lesions were found.Citation9
We reviewed the literature about kidney involvement during WM from 1970 up till now ().
Table 1. Cases of biopsy-proven renal involvement during WM reported in literature since 1975.
We found 32 cases of WM with histologically proven renal involvement, including our report. NS was present in 20 patients (62.5%). Twenty patients presented renal failure defined as serum creatinine >1.5 mg/dL; in eight cases it was superimposed on NS. A number of histological lesions were described; moreover, in five patients the coexistence of different glomerular diseases was observed. Direct infiltration of the kidney by atypical lymphoid cells was present in 17 cases. Most common histologic findings included intracapillary thrombi (five cases) and membranoproliferative glomerulonephritis (five cases) and cryoglobulinemic glomerulonephritis (five cases) followed by AL amyloidosis (four cases), cast nephropathy (four cases), and cryoglobulinemic glomerulonephritis (four cases); three patients presented light-chain deposition disease and three had MCD.
Our patient presented with NS due to MCD and ARF. ARF is an uncommon presentation of WM, usually associated with glomerular deposition of various proteins including IgM, IgG, cryoglobulins, fibrin, light chains, complement, or even amyloid proteins.Citation22 Most common causes of acute renal failure in monoclonal gammopathies, such as hypercalcemia, uric acid nephropathy, dehydration, cast nephropathy, drug-induced ARF, and sepsis, were excluded. We postulate that the severe interstitial lymphocytic infiltrate was the preeminent cause of acute renal failure. MCD is the most common cause of nephrotic syndrome in children, accounting for 90% of cases under the age of 10; in adults of all ages, it is responsible for 15% of NS cases.Citation30 The pathophysiology of MCD remains poorly understood. It is well known that the release of cytokines by T cells plays a key role. More recently, however, accumulating data has pointed toward a strong contribution of B-cell immunity. In particular, an unidentified circulating permeability factor may be directly released from B cells or be secondary to aberrant cross-talk between T and B cells.Citation31 Gilbert et al.Citation32 recently reported long-term remission using rituximab, an anti-CD20 monoclonal antibody, in a child with steroid-dependent MCD, despite failure of multiple other therapies. The child remained in remission, while the CD19 (B-cell) counts were undetectable, but relapsed after 9 months when CD19+ cells returned. The remission of NS in our patient with rituximab seems to emphasize the pathogenetic role of B cells in MCD, although a coincident effect of immunosuppression on both the underlying renal disease and the hematologic disease cannot be excluded. Further studies are needed to better understand the exact role of B cells in the pathogenesis of renal diseases, especially when they occur during hematologic disorders.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Gnemmi V, Leleu X, Provot F, Moulonguet F, Buob D. Cast nephropathy and light-chain deposition disease in Waldenström macroglobulinemia. Am J Kidney Dis. 2012;60(3):487–491
- Gertz MA. Waldenström macroglobulinemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol. 2012;87(5):503–510
- Isaac J, Herrera GA. Cast nephropathy in a case of Waldenström's macroglobulinemia. Nephron. 2002;91(3):512–515
- Haraguchi S, Tomiyoshi Y, Aoki S, Sakemi T. Nephrotic syndrome due to immunologically mediated hypocomplementic glomerulonephritis in a patient of Waldenström's macroglobulinemia. Nephron. 2002;92(2):452–455
- Hory B, Saunier F, Wolff R, Saint-Hillier Y, Coulon G, Perol C. Waldenström macroglobulinemia and nephrotic syndrome with minimal change lesion. Nephron. 1987;45(1):68–70
- Lindström FD, Hed J, Eneström S. Renal pathology of Waldenström's macroglobulinemia with monoclonal antiglomerular antibodies and nephrotic syndrome. Clin Exp Immunol. 1980;41(2):196–204
- Terrier B, Buzyn A, Hummel A, et al. Serum monoclonal component and nephrotic syndrome -- it is not always amyloidosis. Diagnosis: WM complicated by retroperitoneal and renal infiltration and associated with a minimal change disease. Nephrol Dial Transplant. 2006;21(11):3327–3329
- Wong PN, Mak SK, Lo KY, Tong GM, Wong AK. Acute tubular necrosis in a patient with Waldenström's macroglobulinemia and hyperviscosity syndrome. Nephrol Dial Transplant. 2000;15(10):1684–1687
- Morel-Maroger L, Basch A, Danon F, Verroust P, Richet G. Pathology of the kidney in Waldenström's macroglobulinemia. Study of sixteen cases. N Engl J Med. 1970;283(3):123–129
- Martelo OJ, Schultz DR, Pardo V, Perez-stable E. Immunologically-mediated renal disease in Waldenström's macroglobulinemia. Am J Med. 1975;58(4):567–575
- Meyrier A, Simon P, Mignon F, Striker L, Ramée MP. Rapidly progressive (‘crescentic') glomerulonephritis and monoclonal gammapathies. Nephron. 1984;38(3):156–162
- Nakamoto Y, Imai H, Hamanaka S, Yoshida K, Akihama T, Miura AB. IgM monoclonal gammopathy accompanied by nodular glomerulosclerosis, urine-concentrating defect, and hyporeninemic hypoaldosteronism. Am J Nephrol. 1985;5(1):53–58
- Ogami Y, Takasugi M, Soejima M, et al. Waldenström's macroglobulinemia associated with amyloidosis and crescentic glomerulonephritis. Nephron. 1989;51(1):95–98
- Tsuji M, Ochiai S, Taka T, Hishitani Y, Nagareda T, Mori H. Nonamyloidotic nephrotic syndrome in Waldenström's macroglobulinemia. Nephron. 1990;54(2):176–178
- González Anglada MI, González García J, del Arco A, Picazo ML, Quevedo E, Vázquez Rodríguez JJ. Waldenström's macroglobulinemia, cryoglobulinemia type I and membranous glomerulonephritis. Med Clin (Barc). 1991;97(14):539–541
- Veltman GA, van Veen S, Kluin-Nelemans JC, Bruijn JA, van Es LA. Renal disease in Waldenström's macroglobulinemia. Nephrol Dial Transplant. 1997;12(6):1256–1259
- Soetekouw R, Bruijn JA, Hogewind BL, Bieger R. A 66-year-old woman with nephrotic syndrome and an IgM monoclonal gammopathy. Ann Hematol. 1998;76(5):227–230
- Dussol B, Kaplanski G, Daniel L, Brunet P, Pellissier JF, Berland Y. Simultaneous occurrence of fibrillary glomerulopathy and AL amyloid. Nephrol Dial Transplant. 1998;13(10):2630–2632
- Harada Y, Ido N, Okada T, et al. Nephrotic syndrome caused by protein thrombi in glomerulocapillary lumen in Waldenström's macroglobulinemia. Br J Hematol. 2000;110(4):880–883
- Yonemura K, Suzuki T, Sano K, Fujigaki Y, Ikegaya N, Hishida A. A case with acute renal failure complicated by Waldenström's macroglobulinemia and cryoglobulinemia. Ren Fail. 2000;22(4):511–515
- Da'as N, Polliack A, Cohen Y, et al. Kidney involvement and renal manifestations in non-Hodgkin's lymphoma and lymphocytic leukemia: a retrospective study in 700 patients. Eur J Hematol. 2001;67(3):158–164
- Garcia-Pacheco I, Khan A, Venkat KK. Rapidly progressive glomerulonephritis in a patient with Waldenström's macroglobulinemia. Clin Nephrol. 2005;64(5):396–399
- Audard V, Georges B, Vanhille P, et al. Renal lesions associated with IgM-secreting monoclonal proliferations: revisiting the disease spectrum. Clin J Am Soc Nephrol. 2008;3(5):1339–1349
- Colović N, Terzić T, Andelić B, et al. Nephrotic syndrome and acute renal failure in non-Hodgkin lymphoplasmacytic lymphoma. Med Oncol. 2008;25(4):458–461
- Kawano N, Ikeda N, Yoshida S, et al. Successful treatment of cryoglobulinemic glomerulonephritis derived from Waldenström's macroglobulinemia by rituximab-CHOP and tandem high-dose chemotherapy with autologous peripheral blood stem cell transplantation. Int J Hematol. 2010;92(2):391–397
- Kim YL, Gong SJ, Hwang YH, et al. Waldenstrom macroglobulinemia with CD5+ expression presented as cryoglobulinemic glomerulonephropathy: a case report. J Korean Med Sci. 2011;26(6):824–828
- Martina MN, Solé M, Massó E, Pérez N, Campistol JM, Quintana LF. Mixed cryoglobulinemia not related to hepatitis C virus, mesangiocapillary glomerulonephritis and lymphoplasmocytic lymphoma. Nefrologia. 2011;31(6):743–746
- Lee B, Smith RS, Tanphaichitr N, Novak R, Robertson S, Haller NA. Waldenström's macroglobulinemia and nephrotic syndrome with membranous nephropathy. Scand J Urol Nephrol. 2011;45(6):473–477
- Pérez NS, Garcia-Herrera A, Rosiñol L, et al. Lymphoplasmacytic lymphoma causing light chain cast nephropathy. Nephrol Dial Transplant. 2012;27(1):450–453
- Yang T, Nast CC, Vo A, Jordan SC. Rapid remission of steroid and mycophenolate mofetil (mmf)-resistant minimal change nephrotic syndrome after rituximab therapy. Nephrol Dial Transplant. 2008;23(1):377–380
- Takei T, Nitta K. Rituximab and minimal change nephrotic syndrome: a therapeutic option. Clin Exp Nephrol. 2011;15(5):641–647
- Gilbert R, Hulse E, Rigden S. Rituximab therapy for steroid dependent minimal change nephrotic syndrome. Pediatr Nephrol. 2006;21:1698–1700