Abstract
Various reasons such as malignancies and chronic infections may cause weight loss in kidney transplant patients. In this report, iron overload as a rare cause of weight loss in a kidney transplant patient is presented. Forty-seven-year-old male patient who transplanted from a deceased donor 5 years ago was hospitalized because of 20 kg of weight loss. In medical history, he had history of hemodialysis for 89 months and received 100–300 mg of intravenous iron therapy per week before transplantation and transfused eight units of blood. In physical examination, weight and height were 45 kg and 185 cm, respectively. Respiratory and cardiac auscultation was normal. Laboratory results revealed as follow: glucose 76 mg/dL, urea 60 mg/dL, creatinine 1.35 mg/dL, aspartate aminotransferase 74 U/L, alanine aminotransferase 77 U/L, C-reactive protein 2.59 mg/dL, albumin 3.3 g/dL, globulin 3.4 g/dL, white blood cells 3200/mm3, hemoglobin 13.1 g/dL and platelets 190,000/mm3. Chest and abdominal tomography didn’t reveal any pathology. Portal Doppler ultrasound showed signs of early cirrhosis. Viral and autoimmune hepatitis markers were negative. Ferritin was 5300 ng/mL and transferrin saturation was 82%. In liver biopsy, hemosiderosis was diagnosed and heterozygous H63D gene mutation was detected. Totally, 19 units of phlebotomy were performed. Liver function tests and serum ferritin decreased gradually. At outpatient follow-up in 6 months, he returned to former weight. In conclusion, there can be several causes of weight loss in kidney transplant patients. Iron overload can come across as a rare cause of weight loss. In these patients, ferritin levels should be checked and diagnosis should be clarified by liver biopsy and gene mutation analysis.
Introduction
Weight loss is an important symptom and finding that concern clinicians in kidney transplant patients. Firstly, malignancy and chronic infections such as tuberculosis must be suspected.Citation1 However, some rarely seen diseases such as iron overload may be missed. The best indicators of iron overload are the elevation in plasma ferritin level and transferrin saturation.Citation2 However, hyperferritinemia is commonly found not only due to iron overload but also chronic inflammatory status, malnutrition or neoplasias in patients with chronic kidney disease.Citation3 If iron overload was not diagnosed and treated earlier, long-term irreversible complications such as diabetes, liver cirrhosis, and even hepatocellular cancer that affected the survival adversely might be result.Citation4 In this article, a case of iron overload that caused weight loss in a renal transplant patient is aimed to present.
Case
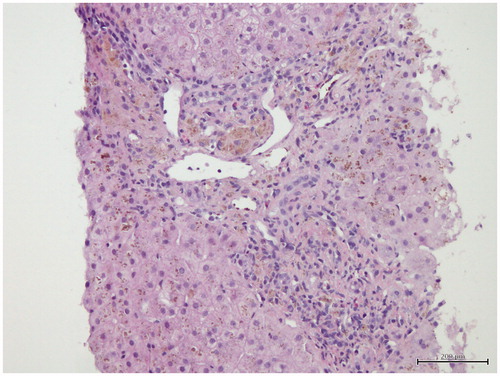
Forty-seven-year-old male patient who was followed for 5 years because of deceased renal transplantation and complaining about weight loss of 20 kg within the last 2 months was hospitalized for tests. His maintenance immunosuppressive therapy was mycophenolate sodium and prednisolone. In medical history, he had history of hemodialysis for 89 months and received 100–300 mg of intravenous (IV) iron therapy per week before transplantation and eight units of blood transfusion. Furthermore, patient had been performed the internal cardiac defibrillator (ICD) implantation for primary prophylaxis due to recurrent ventricular premature beats 3 years ago. In the physical examination, body weight and height were 45 kg and 185 cm, respectively. His weight was 65 kg 2 months ago. Respiratory and cardiac auscultation was normal. Laboratory results revealed as follow: glucose 76 mg/dL, urea 60 mg/dL, kre 1.35 mg/dL, aspartate aminotransferase (AST) 74 U/L, alanine aminotransferase (ALT) 77 U/L, C-reactive protein 2.59 mg/dL, albumin 3.3 g/dL, globulin 3.4 g/dL, white blood cells 3200/mm3, hemoglobin 13.1 g/dL and platelets 190,000/mm3. Erythrocyte sedimentation rate was 18 mm/h. Thyroid function tests showed the following free T3 2.09 pg/mL (2.3–4.2), free T4 1.03 ng/dL (0.89–1.76) and TSH 7.89 μıu/mL (0.35–5.5). Chest X-ray was normal. Thoracic and abdominal computerized tomography (CT) which was performed to determine any malignancy did not reveal evidence of any malignancy, but it showed minimal fluid in pelvic and perihepatic area. Therefore, portal Doppler ultrasound was performed to determine if there is portal hypertension. The portal Doppler ultrasound had showed following: liver slightly larger in size and parenchymal echo slightly elevated. The spleen measured 13.5 cm. Diameter of portal vein, splenic vein and superior mesenteric vein (SMV) was 10, 9 and 9 mm, respectively and current directions were hepatopetal. Diameter of coronary vein was 5.5 mm and flow was hepatofugal. Minimal free fluid was detected in the pelvis between bowel loops. These Doppler findings were signs of early liver cirrhosis. Therefore, upper gastrointestinal endoscopy was performed and was found normal. Prothrombin time was 14 s. Markers for viral and autoimmune hepatitis were negative. Ferritin level was 5300 ng/mL and transferrin saturation ratio was 82%. Elevated ferritin level, high transferrin saturation ratio and moderate elevation of liver function tests suggested that diagnosis might be iron overload. In the history of the patient, there was not lot of blood transfusion that could cause secondary hemochromatosis. But, he had received 100–300 mg of IV iron therapy per week in HD period. The patient underwent liver biopsy with the pre-diagnosis of hemosiderosis. In addition, the HFE gene mutation was examined in terms of hereditary hemochromatosis (HH). In liver biopsy, iron accumulation was demonstrated in hepatocytes, parenchymal and portal Kupffer cells ( and ). Minimal to moderate portal and periportal fibrosis was also seen (). Heterozygous H63D gene mutation was detected in HFE genes. Intermittent phlebotomy according to complete blood count was performed after the patient had been discharged from the hospital. In total, 19 units of phlebotomy were performed. The hemoglobin of patient remained stable. Liver function tests and serum ferritin decreased gradually by phlebotomy (). At outpatient follow-up period of 6 months, the patient returned to his former weight and the last ferritin level was 1478 ng/mL at eighth month of treatment.
Figure 2. There is marked iron deposition (Grade 3) that is most prominent in portal macrophages and zone 1 hepatocytes and Kupffer cells (Perls stain ×200).
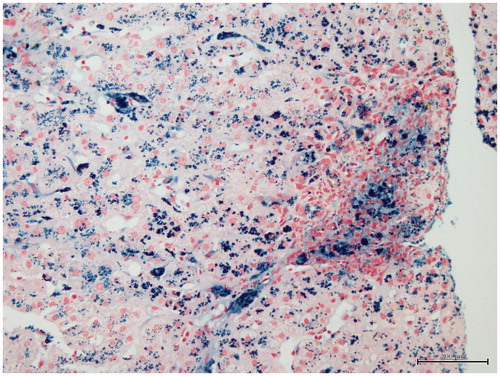
Table 1. Treatment and blood parameters of patient.
Discussion
Weight loss is one of the major symptoms that physicians concern not only in renal transplant patients but also in normal individuals. There are several causes of weight loss including endocrine diseases such as diabetes mellitus (DM), hyperthyroidism and adrenal insufficiency; infectious diseases such as tuberculosis and human immunodeficiency virus (HIV); gastrointestinal disorders such as malabsorption and inflammatory bowel disease; psychiatric disorders such as anorexia nervosa; severe heart, lung and kidney diseases and malignancies.Citation5 All causes of weight loss should be examined in detail.
HH is one of the rare causes of weight loss. But diagnosis of HH is difficult because iron overload may be secondary to multiple blood transfusions and/or IV iron therapy. If the diagnosis was either secondary hemochromatosis (iron overload) or HH, the treatment would be the same, therapeutic phlebotomy.
Dialysis patients usually receive IV iron therapy because iron deficiency and iron mobilization defect limit the erythropoietic response to erythropoiesis-stimulating agents.Citation6 However, IV iron preparations are prescribed routinely without enough attention in some cases. This careless application can lead to iron overload and/or intensification of oxidative stress and inflammation.Citation7 In addition, this approach may contribute to the cardiovascular and infectious mortality that are the two main causes of death in dialysis patients by affecting cardiovascular and immune system.Citation7 Although guidelines recommend IV iron therapy to HD patients with serum ferritin values as high as 500–900 ng/mL, it was found that serum ferritin levels over 124 ng/mL were associated with increased bone marrow iron load in a study.Citation8 Therefore, clinicians should be more careful in terms of IV iron use in dialysis patients because it may cause iron overload, oxidative stress and inflammation.
Hyperferritinemia is commonly found in HD patients independent of the hemoglobin level and generally associated with chronic inflammatory status as well as malnutrition and neoplasias.Citation3 However, after kidney transplantation, inflammation and consequent hyperferritinemia will not be observed because uremic environment are eliminated. In fact, it had been shown that iron overload was the cause of hyperferritinemia in a previously presented kidney transplant patient by rule out of other causes.Citation3 Excessively used IV iron can lead to iron overload in HD patients undergoing kidney transplantation, especially, in men or postmenopausal women because iron will not be lost in these patients. Therefore, hyperferritinemia should be evaluated more carefully in men or postmenopausal women.
Although iron accumulation may be asymptomatic in some patients, in others many symptoms and signs such as, fatigue, weakness, skin pigmentation, DM, liver function impairment, arthralgia, impotence in men, cardiomegaly, and cardiac conduction defects can be seen.Citation9 Deterioration in liver function tests, fatigue and weight loss were present in our patient. His medical history had also the ICD implantation due to ventricular premature beats (conduction defect) 3 years ago. However, at that time he had not been examined in terms of iron accumulation. Probably the conduction defect could be caused by secondary hemochromatosis. There were no other symptoms or signs of iron overload in our patient.
Endocrine diseases like hyperthyroidism and adrenal insufficiency can cause weight loss.Citation5 Our patient had hypothyroidism. So that we did not think that thyroid dysfunction could cause weight loss. We have not determined basal cortisol level in terms of adrenal insufficiency in our patient because he was under treatment of prednisolone and he had no findings of adrenal insufficiency like hypoglycemia and hypotension.
In secondary hemochromatosis, weight loss especially occurs when disorders such as DM and cirrhosis appear due to iron accumulation in the parenchyma of organs. Rarely, patients may present with constitutional symptom without a serious organs involvement.Citation10 Although our patient had deterioration in liver function tests, he did not have evidence of advanced liver cirrhosis. Moderate thyroid dysfunction was also detected but there was no diabetes.
Therapeutic phlebotomy is the simplest, cheapest and most effective way of elimination of iron deposition in non-anemic patients.Citation11 About 200–250 mg of iron can be removed with each 500 mL of whole blood. Iron is lost via the red cells by phlebotomy and subsequently iron pass into circulation from its stores and used for the production of hemoglobin. Serum ferritin level was very high and there was not anemia in our patient. After applying therapeutic phlebotomy, symptoms such as weight loss and fatigue disappeared.
Wagner et al.Citation12 had showed DM and weight loss due to HH by presenting a case aged 60 years. In spite of the treatment by therapeutic phlebotomy, some heavy conditions (cirrhosis, DM) were irreversible. There were two differences between our case and that case. First, our case was a kidney transplant patient who had history of 89 months of dialysis, 100–300 mg of IV iron therapy per week and eight units of blood transfusion prior to transplantation. Second, our patient was diagnosed at an earlier age before the occurrence of irreversible changes such as DM and advanced liver cirrhosis.
In conclusion, there can be several causes of weight loss in kidney transplant patients. Secondary hemochromatosis due to iron overload can come across as a rare cause of weight loss. In these patients ferritin levels should be checked and, if necessary, the diagnosis should be clarified by liver biopsy and gene mutation analysis. Therapeutic phlebotomy is the simplest, cheapest and most effective treatment in these patients.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Bouras EP, Lange SM, Scolapio JS. Rational approach to patients with unintentional weight loss. Mayo Clin Proc. 2001;76(9):923
- Beaton MD, Adams PC. Treatment of hyperferritinemia. Ann Hepatol. 2012;11(3):294–300
- Furić-Cunko V, Basić-Jukić N, Jurić I, Kes P. Hyperferritinemia in a kidney transplant recipient. Acta Clin Croat. 2011;50(2):245–248
- Fargion S, Valenti L, Fracanzani AL. Beyond hereditary hemochromatosis: new insights into the relationship between iron overload and chronic liver diseases. Dig Liver Dis. 2011;43(2):89–95
- Evans AT, Gupta R. Approach to the patient with weight loss. Available at: www.uptodate.com. Accessed October 21, 2012
- Hörl WH. Clinical aspects of iron use in the anemia of kidney disease. J Am Soc Nephrol. 2007;18(2):382–393
- Vaziri ND. Understanding iron: promoting its safe use in patients with chronic kidney failure treated by hemodialysis. Am J Kidney Dis. 2013;61(6):992–1000
- Mirahmadi KS, Paul WL, Winer RL, et al. Serum ferritin level determinant of iron requirement in hemodialysis patients. JAMA. 1977;238(7):601–603
- Bacon BR, Adams PC, Kowdley KV, Powell LW. Anthony S. Tavill. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(1):328–343
- Niederau C, Strohmeyer G, Stremmel W. Epidemiology, clinical spectrum and prognosis of hemochromatosis. Adv Exp Med Biol. 1994;356:293--302
- Barton JC, McDonnell SM, Adams PC, et al. Management of hemochromatosis. Hemochromatosis Management Working Group. Ann Intern Med. 1998;129:932--939
- Wagner R, Tabák A, Bálint Z, Andrikovics H, Tordai A, Demeter J. A case of hemochromatosis diagnosed in a middle-aged patient with diabetes mellitus. Orv Hetil. 2006;147(25):1179–1184