Abstract
This study aimed to assess the effectiveness and safety of moderate-dose glucocorticoids (GCs) with mechanical ventilation as salvage therapy for renal transplant recipients with severe pneumonia, which was non-responsive to conventional treatment. A retrospective study was conducted involving renal transplant recipients diagnosed with severe pneumonia and did not respond to conventional treatment. All immunosuppressants were then completely withdrawn, and the patients were initially administered with methylprednisolone at doses of 2.0–2.5 mg/kg/day once every 12 h. This dosage was continued until oxygenation improved, and the treatment was gradually tapered (by 20 mg every 2–3 days) to the previous maintenance dosage. Ten patients were recruited from year 2008 to 2012. Two patients who underwent emergency endotracheal intubation were intubated on days 3 and 8, respectively, another one died from recurrent pneumothorax. The mean PaO2/FiO2 of the nine survivors was significantly increased by the increasing treatment duration; whereas the lung injury scores (LIS) and the sequential organ failure assessment (SOFA) score were both significantly decreased. The use of moderate-dose GCs may play a role as salvage therapy for renal transplant recipients with severe pneumonia. However, further study with larger trials to is needed.
Introduction
Pulmonary infection remains a significant problem in the general population. Although severe pneumonia is rare after kidney transplantation, exposure to induction and long-term maintenance immunosuppressive therapy carries a high risk of infection and increases the risk for acute respiratory distress syndrome (ARDS).Citation1 Among patients receiving kidney transplants, the 28-day mortality in subjects with ARDS is 52.1%, which is much higher than that in the general population.Citation2,Citation3 An excessive lung inflammatory response that is associated with microbial invasion can lead to the rapid progression of ARDS despite the administration of broad-spectrum anti-infective agents and the dramatic tapering of immunosuppressants in renal transplant recipients. Therefore, other underlying factors may be responsible for the poor outcomes. The administration of glucocorticoids (GCs) can inhibit the expression and activity of many cytokines involved in inflammatory response and may decrease mortality in patients with severe pneumonia.Citation4,Citation5 However, the routine use of GCs for patients with ARDS remains controversial.Citation6–8 The role and efficacy of GCs have not been well established in solid organ transplant recipients with ARDS because these patients have been universally excluded from randomized trials on account of their immunocompromised state.Citation9
We have taken an aggressive diagnostic and therapeutic approach for renal transplant recipients with severe pneumonia. Despite the administration of anti-infective agents and the reduction of immunosuppressant doses, some patients still rapidly progress to widespread pulmonary infiltrates, if not the complete “white-out” of both lung fields within a short period. Non-invasive ventilation (NIV) at an early stage of ARDS reduces the need for endotracheal intubation as well as the incidence of complications associated with tracheal intubation and mechanical ventilation in immunocompromised patients.Citation10,Citation11 However, the high rate of NIV failure suggests that the use of NIV alone may not be sufficient to solve this problem.Citation12,Citation13
Therefore, we conducted a retrospective study to investigate the effectiveness and safety of moderate-dose GCs in renal transplant recipients on mechanical ventilation, including NIV or invasive ventilation, as salvage therapy for severe pneumonia when the infection is unresponsive to conventional treatment.
Methods
Patients
Over a 4-year period between 2008 and 2011, 165 transplant recipients in their first year post-renal transplantation were enrolled in an ongoing surveillance study as having a high risk for pneumonia. All renal transplant recipients with severe pneumonia were admitted to 10-bed mixed intensive care unit (ICU) of the Zhongshan Hospital, Fudan University (China). It is worth mentioning that currently ICU care is being provided by the intensivists with experience in organ transplantations. Severe pneumonia was defined as a pulmonary infection that required admission to the ICU. The need for ICU care is suggested by either one of two major criteria (i.e., mechanical ventilation or shock) or at least three minor criteria: a respiratory rate, ≥30 breaths/min; a PaO2/FiO2 ratio, ≤250; multilobar infiltrates; confusion; blood urea nitrogen, ≥20 mg/dL (blood urea, 7 mmol/L); leukopenia; thrombocytopenia; hypothermia; or hypotension requiring fluid support.Citation14 The patients were initially administered with conventional treatment (see below). The study was approved by the Ethics Committee of Zhongshan Hospital, and no informed consent was required for the retrospective study, as approved by our local ethics committee.
Conventional treatment
All patients with severe pneumonia underwent aggressive diagnostic approaches, including fiberoptic bronchoscopy with bronchoalveolar lavage as well as measurements of serum antibodies against cytomegalovirus, Epstein–Barr virus, Mycobacterium tuberculosis, and Mycoplasma. Immunosuppressants were drastically reduced in all patients for the first 2–3 days after the patient’s initial presentation and oxygenation. Empirical antibiotic therapy included moxifloxacin, meropenem, ganciclovir, and trimethoprim/sulfamethoxazole (TMP-SMX). If fungal infection was suspected, antifungal therapy was initiated. The dosages of all drugs were adjusted based on the allograft function. The heart rate (HR), blood pressure (BP), respiratory rate (RR), and arterial oxygen saturation (SaO2) were continuously monitored in all patients. Conservative fluid management was routinely performed in our institution. Other aspects of care were managed following a pre-established protocol addition to the administration of anti-infective agents; the said protocol followed guidelines for fluid resuscitation, blood glucose control, nutrition support, sedation, and analgesia, as well as gastrointestinal and thromboembolic prophylaxis.Citation15
NIV protocol
NIV was considered the first-line treatment if the arterial oxygen tension/inspired oxygen fraction (PaO2/FiO2 ratio) was <200 mmHg while breathing oxygen delivered by a conventional face mark at a maximum concentration.
When NIV was ordered, the head of the bed was kept elevated at a 30°–60° angle, and the patients were not sedated. All patients used a face mask during the duration of the study (ZS-MZ-A Face Mask; Shanghai Zhongshan Medical Technology, Shanghai, China). A seal connector on the dome of the mask was used for the passage of the nasogastric tube. When the mask was first applied to the patient’s face, the patient or bedside staff held it in place until the patient accepted the application. Pressure support ventilation (PSV) with positive end-expiratory pressure (PEEP) was used. PSV was increased in increments of 2–3 cm H2O until it reached 15 cm H2O to obtain a tidal volume of 6 mL/kg and a respiratory rate of ≤25 breaths/min. The PEEP was increased in increments of 2–3 cm H2O until it reached 10 cm H2O to ensure that the SaO2 was >90% and the lowest possible amount of supplemental oxygen was used. The ventilator settings were then adjusted based on pulse oximetry and measurements of arterial blood gases. The bedside staff recorded information regarding the time of NIV initiation, physiological parameters (RR, HR, BP, and temperature), neurological status, and the arterial blood gases immediately after NIV initiation.
The following predetermined criteria were used for endotracheal intubation: (1) patients who failed to maintain a PaO2/FiO2 of 100 mmHg despite optimal standard medical management or NIV with an RR of >30 breaths/min and a blood pH <7.3, (2) patients with hemodynamic instability, (3) patients unable to protect the airway (a Glasgow Coma Scale score of 8 or lower) and (4) patients unable to clear airway secretions. The final decision for intubation was made by the attending physician of the ICU. Ventilator management incorporated the tidal volume and PEEP, according to the guidelines of ARDSnet.Citation16
NIV was continuously maintained until oxygenation and the clinical status were improved. When the PEEP settings decreased to 5 cm H2O, the patient was assessed daily while breathing supplemental oxygen without mechanical ventilator support for 30 min. NIV was discontinued if the patient maintained an RR ≤25 breaths/min and a PaO2 >80 mmHg without activation of the accessory muscles of respiration.
Salvage GC therapy
Patients were considered candidates for salvage GC therapy if they met the following criteria: (1) pulmonary infiltrates and progressive worsening of hypoxemia according to high-resolution computed tomography (HRCT) or chest X-rays despite conventional treatment, (2) evidence of progressive respiratory failure with a PaO2/FiO2 < 200 and (3) mechanical ventilator support to maintain pulmonary oxygenation, including NIV or invasive ventilation. All immunosuppressants were withdrawn. Written informed consent was obtained from all patients before GC administration. The patients were initially administered with methylprednisolone at 2.0–2.5 mg/kg/day once every 12 h. This dosage was continued until oxygenation improved, followed by gradual tapering (via a 20 mg reduction every 2–3 days). When the dosage of intravenous methylprednisolone was reduced to 1.0 mg/kg/day, calcineurin inhibitors were started at a low dose. The route of GC administration was changed from intravenous to enteral when the dose of intravenous methylprednisolone was reduced to 40 mg/day. This dosage was gradually reduced to the previous maintenance dosage over 1–2 weeks. The patients were followed up until 31 December 2012.
Statistical analysis
Summary data were collected and expressed as percentages. Normally distributed data were reported as the means ± SD, whereas non-normal data were reported as the medians (first to third quartiles). The overall time courses of PaO2/FiO2, the lung injury score (LIS), and the sequential organ failure assessment (SOFA) score were compared using the Student’s t-test. Values with p < 0.05 were considered significant. The cumulative probability of remaining on mechanical ventilation was estimated via the Kaplan–Meier method. All statistical calculations were performed by using SPSS for Windows (version 11.5; SPSS Inc., Chicago, IL). Graphs were created using Graph Pad Prism (version 5, Graphpad Software Inc., La Jolla, CA).
Results
Patient characteristics
Between 2008 and 2011, 15 recipients with severe pneumonia were admitted to the ICU in the first year post-renal transplantation. Five patients recovered under conventional therapy, including treatment with anti-infective agents and the adjustment of immunosuppressants. Although these patients developed mild hypoxia during treatment, the level of arterial oxygen saturation (measured by oximetry) was maintained above 93% via oxygen supplementation, as delivered by face mask. Ten patients treated with conventional therapy continued to deteriorate, developed arterial hypoxemia, and required mechanical ventilator support (). All 10 patients met the ARDS criteriaCitation17 and received moderate-dose GCs as salvage therapy. shows the main characteristics of the 10 patients. The baseline immunosuppression regimens included cyclosporin A (CsA), tacrolimus (TAC), mycophenolate mofetil (MMF), and prednisone (Pred) in the following combinations: CsA + MMF + Pred (four patients) and TAC + MMF + Pred (six patients). During the initial mechanical ventilation, the mean patient body temperature was 38.5 °C (38.3–38.7 °C). Eight patients (80%) had a temperature higher than 38 °C, and three (30%) had a cough. The average acute physiology and chronic health evaluation II (APACHE II) score of the patients was 16 (ranging from 14 to 20). The baseline laboratory findings on the 10 patients are listed in .
Figure 1. Flow chart of the studied patients. i-MV, invasive mechanical ventilation; NIV, non-invasive ventilation; ARDS, acute respiratory distress syndrome; GC, glucocorticoid.
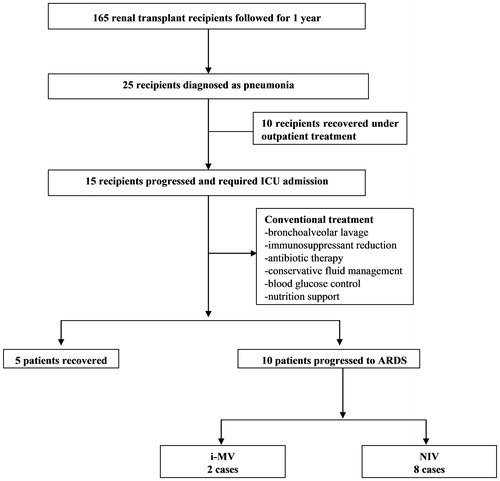
Table 1. Demographics and clinical characteristics of the enrolled patients.
Table 2. Baseline levels of 10 renal transplant patients with severe pneumonia.
Primary pathogen and nosocomial infection
All patients underwent a bronchoscopic bronchoalveolar lavage to identify possible pathogens. Three patients had a definitive diagnosis of pneumonia caused by Pneumocystis carinii (patient no. 6), cytomegalovirus (patient no. 8), and Mycoplasma (patient no. 10). No pathogens were isolated from the remaining seven patients with pneumonitis-induced respiratory failure.
During ICU treatment, nosocomial infections were actively observed to detect for GC-related secondary infections. Patient no. 4 developed ventilator-associated pneumonia (VAP), as caused by Pseudomonas aeruginosa, and was treated with cefepime. Patient no. 6 developed a central venous catheter-related bloodstream infection (CRBSI) during extracorporeal membrane oxygenation (ECMO). Teicoplanin was administered to treat the infection, which was caused by methicillin-resistant Staphylococcus aureus (MRSA).
Intubation and mortality
Two patients underwent emergency endotracheal intubation because of severe hypoxia (SpO2 <75%) and hemodynamic instability. These patients were extubated on days 3 and 8, respectively, and subsequently received NIV as a sequential weaning therapy. Both patients were successfully weaned from NIV and converted to oxygen therapy with a mask on days 8 and 16, respectively.
Among the eight patients receiving NIV as initial respiratory support, patient no. 6 abruptly developed respiratory distress on day 3, and a chest radiograph indicated a left pneumothorax. Patient no. 6 then developed severe hypoxemia, a hemodynamic crisis, and extensive subcutaneous emphysema from head to trunk. To oxygenate the patient’s blood and reduce the carbon dioxide concentration, venovenous ECMO was performed. Although the patient was weaned from ECMO therapy and underwent 6 h of mechanical ventilation daily, he died of a recurrent pneumothorax.
Changes in PaO2/FiO2 ratio, LIS, SOFA score, and time course of mechanical ventilation
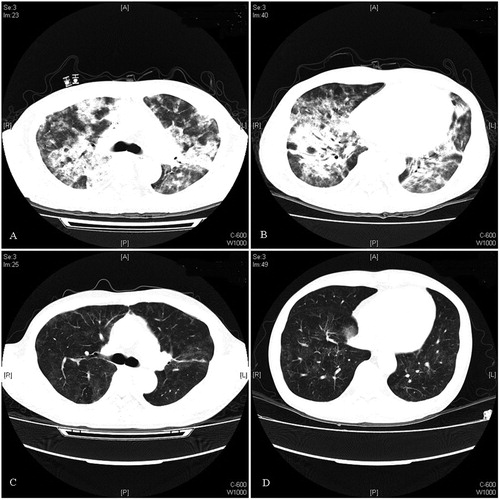
During the initial mechanical ventilation, two patients were diagnosed with severe ARDS (PaO2/FiO2 < 100), whereas eight patients had moderate ARDS (100 < PaO2/FiO2 < 200). The mean PaO2/FiO2 ratio of the nine survivors was 139 ± 41 at the baseline, which increased significantly to 195 ± 39 (p < 0.001) on day 3, 252 ± 45 (p = 0.008) on day 5, and 330 ± 96 (p = 0.013) on day 7 (). LIS was used to assess the extent of acute pulmonary damage, whereas the SOFA score quantified organ dysfunction and clinical severity. The treatment significantly reduced the LIS and SOFA scores. Among the survivors, the LIS decreased from 2.6 ± 0.6 on day 1 to 2.2 ± 0.5 (p < 0.001) on day 3, 1.8 ± 0.3 (p = 0.015) on day 5, and 1.5 ± 0.4 (p = 0.048) on day 7 (). The SOFA score at the beginning of treatment was 5.2 ± 3.3, which decreased to 4.6 ± 3.2 (p = 0.004) on day 3, 3.9 ± 3.0 (p = 0.022) on day 5, and 3.4 ± 3.3 (p = 0.035) on day 7 (). Pulmonary changes were monitored with HRCT or chest X-rays. The distinct improvement in the chest computed tomography scan of patient no. 5 is shown in .
Figure 2. Changes in the (A) PaO2/FiO2 ratio, (B) LIS, (C) SOFA scores, and (D) cumulative time on mechanical ventilation of the nine surviving patients during the first 7 days of treatment. SOFA score, Sequential Organ Failure Assessment score; MV, mechanical ventilation.
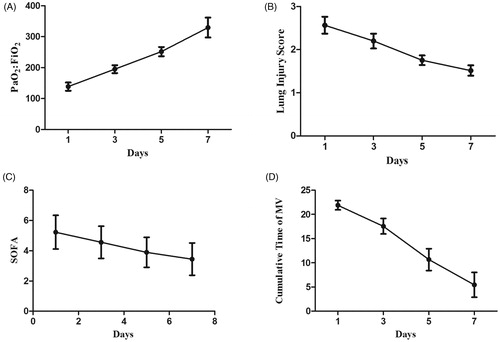
Figure 3. Chest CT of patient no. 5: (A and B) chest CT scans showing the presence of symmetrically distributed infiltrations and (C and D) follow-up chest CT scan after 3-week treatment showing the marked radiological improvement of the same infiltrations.
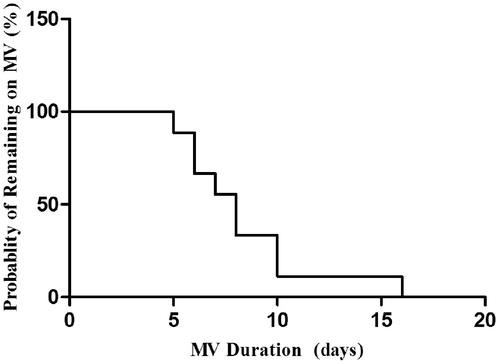
The cumulative time on mechanical ventilation of the nine surviving patients during the first week of treatment was showed in . By day 7, four patients who received NIV as initial respiratory support were completely weaned from NIV. One patient received ECMO treatment, whereas three received intermittent NIV support for an average of 10 h. The probability of the survivors remaining on mechanical ventilation is shown in .
Discussion
The administration of GCs is a potential treatment for severe pneumonia because of their anti-inflammatory properties.Citation18 Early trials using time-limited high-dose corticosteroids failed to benefit patient survival and indicated a possible risk to ARDS patients.Citation19–21 However, prolonged low-to-moderate-dose corticosteroid regimens may ameliorate pulmonary inflammation and reduce the morbidity and mortality of ARDS.Citation9,Citation22 To date, the optimal initial dose of methylprednisolone in patients with early severe ARDS is 1 mg/kg/day, and patients with unresolved ARDS have been benefited from treatment with methylprednisolone (2 mg/kg/day) initiated before day 14.Citation6,Citation23 These studies were primarily based on randomized trials among non-immunosuppressed populations. So far, no direct evidence has been showed that GCs treatment can decrease the mortality in immunocompromised patients with ARDS. To the best of our knowledge, this study is the first case series of renal transplant recipients with ARDS that evaluates patient response to moderate-dose corticosteroid treatment. Our preliminary work finds that using moderate-dose corticosteroids as salvage therapy significantly improved oxygenation and reduced the LIS and SOFA scores during the first week of treatment.
We administered GCs in this study for three main reasons: (1) Renal transplant recipients commonly receive low-dose GCs as part of immunosuppressive treatment protocols. Therefore, latent adrenal insufficiency may already exist in these patients. Critical illness-related corticosteroid insufficiency (CIRCI) is defined as inadequate cellular corticosteroid activity given the severity of the patient’s illness; CIRCI may lead to the persistent elevation of pro-inflammatory mediators in ARDS over time.Citation24,Citation25 (2) Previous studies have evaluated the effect of GC treatment on ARDS. Although the study designs, dosing strategies, and therapy duration significantly varied among these studies, all consistently demonstrated that GC treatment significantly improves PaO2/FiO2, as well as significantly reduces markers of systemic inflammation and the duration of mechanical ventilation.Citation9,Citation22,Citation26 (3) The concern regarding reduced immunosuppressant dosage or even withdrawal is primarily related to the fear of future allograft rejection. The use of GCs as substitutes minimizes the risk of acute rejection. We did not observe any clinically evident acute rejection episodes during hospitalization, although the administration of immunosuppressive agents was aggressively reduced for an average of 14 days. We followed up our patients for a mean duration of 8 months and did not observe any acute allograft rejection.
Based on our single-center experience, mechanical ventilation increased mortality in organ transplant recipients. In this study, the initial treatment was considered ineffective if bilateral infiltrates and progressively worsening hypoxemia were observed. Mechanical ventilation was likewise required. Antonelli et al.Citation27 studied solid organ transplant recipients with hypoxemic acute respiratory failure. Among 20 patients receiving NIV, 4 (20%) required endotracheal intubation, and ICU mortality was 20%. Hilbert et al.Citation28 similarly examined 26 immunocompromised patients receiving NIV as initial respiratory support. About 12 (46%) patients required endotracheal intubation, 10 (38%) died in the ICU, and 13 (50%) died in the hospital. In our case series, only 1 (1/8) patient failed NIV because of ventilator-induced lung injury, endotracheal intubation, and ECMO. The ICU and hospital mortality observed in the present study cohort were relatively lower, as compared with the previous publications (). We hypothesize that GC treatment inhibits overactive systemic inflammation, thereby increasing the NIV success rate and ensuring early weaning from mechanical ventilator support.
Table 3. Comparison of baseline characteristics and outcome events in immunocompromised patients receiving NIV in three studies.
Calcineurin inhibitors, such as CsA and FK506, as well as antimetabolites, such as MMF, are commonly used immunosuppressants; these drugs significantly affect both the innate and acquired immunity.Citation29–31 The possibility that GC administration would place the patient at increased risk for severe infectious complications should be investigated. In a previous randomized double-blind trial to evaluate the effectiveness of early methylprednisolone therapy for patients with pulmonary failure, early infectious complications occurred in 30 steroid-treated patients (77%) and 18 placebo-treated patients (43%). GCs failed to improve pulmonary function and were associated with the increased infection rate. Methylprednisolone was administered over a short time (48 h) and at an extremely high daily dose (up to 120 mg/kg/day) in this trial conducted in the 1980s.Citation19 By contrast, a more recent systematic review of prolonged GC treatment (PGCT) at low-to-moderate doses did not identify an association with the increased rate of major complications, including nosocomial infections and neuromyopathy.Citation32 Three previous trials have reported that “stress-dose” GCs reduce the acquisition of secondary infections.Citation6,Citation9,Citation33 The cumulative evidence indicates that PGCT can improve innate immune response of ARDS patients by downregulating the life-threatening systemic inflammation and providing a less favorable environment for the intracellular and extracellular growth of bacteria.Citation34–36 In the present study, only two nosocomial infections occurred during treatment: VAP caused by P. aeruginosa and CRBSI caused by MRSA. However, immunocompromised patients receiving PGCT should still be closely monitored to prevent potential secondary infections.
Some limitations of our study should be mentioned. The first was the relatively small number of patients, which prevents us from drawing conclusions on the real benefits of GC treatment in immunocompromised patients. Second, the study lacked a control group, and the possibility that the favorable outcome of our patients was related to the natural course of ARDS cannot be excluded. Although significant improvement in oxygenation and radiography was observed after GC treatment in this study, we cannot definitively conclude that this was entirely due to the GC treatment. Finally, all the recipients were recruited within the first year post-renal transplantation, therefore, an appropriately powered multicenter trial including a much higher number of patients is necessary.
Conclusions
The use of moderate-dose GCs with mechanical ventilation may play a role as salvage therapy for highly selected renal transplant recipients with severe pneumonia. Our findings merit further study with larger trials to evaluate the effect of GC treatment on clinical outcomes.
Declaration of interest
The authors have no conflict of interest.
References
- Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614
- Shorr AF, Abbott KC, Agadoa LY. Acute respiratory distress syndrome after kidney transplantation: epidemiology, risk factors, and outcomes. Crit Care Med. 2003;31:1325–1330
- Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533
- Sibila O, Agusti C, Torres A. Corticosteroids in severe pneumonia. Eur Respir J. 2008;32:259–264
- Lee HS, Lee JM, Kim MS, Kim HY, Hwangbo B, Zo JI. Low-dose steroid therapy at an early phase of postoperative acute respiratory distress syndrome. Ann Thorac Surg. 2005;79:405–410
- Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684
- Peter JV, John P, Graham PL, Moran JL, George IA, Bersten A. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ. 2008;336:1006–1009
- Agarwal R, Nath A, Aggarwal AN, Gupta D. Do glucocorticoids decrease mortality in acute respiratory distress syndrome? A meta-analysis. Respirology. 2007;12:585–590
- Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131:954–963
- Hill NS, Brennan J, Garpestad E, Nava S. Noninvasive ventilation in acute respiratory failure. Crit Care Med. 2007;35:2402–2407
- Piastra M, De Luca D, Pietrini D, et al. Noninvasive pressure-support ventilation in immunocompromised children with ARDS: a feasibility study. Intensive Care Med. 2009;35:1420–1427
- Nava S, Schreiber A, Domenighetti G. Noninvasive ventilation for patients with acute lung injury or acute respiratory distress syndrome. Respir Care. 2011;56:1583–1588
- Bello G, De Pascale G, Antonelli M. Noninvasive ventilation for the immunocompromised patient: always appropriate? Curr Opin Crit Care. 2012;18:54–60
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl. 2):S27–S72
- Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327
- Girard TD, Bernard GR. Mechanical ventilation in ARDS: a state-of-the-art review. Chest. 2007;131:921–929
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723
- Weigelt JA, Norcross JF, Borman KR, Snyder WH III. Early steroid therapy for respiratory failure. Arch Surg. 1985;120:536–540
- Bernard GR, Luce JM, Sprung CL, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987;317:1565–1570
- Luce JM, Montgomery AB, Marks JD, Turner J, Metz CA, Murray JF. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis. 1988;138:62–68
- Meduri GU, Headley AS, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998;280:159–165
- Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36:1937–1949
- Marik PE. Critical illness-related corticosteroid insufficiency. Chest. 2009;135:181–193
- Kwon YS, Suh GY, Jeon K, et al. Serum cytokines and critical illness-related corticosteroid insufficiency. Intensive Care Med. 2010;36:1845–1851
- Confalonieri M, Urbino R, Potena A, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171:242–248
- Antonelli M, Conti G, Bufi M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA. 2000;283:235–241
- Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344:481–487
- Ma W, Mishra S, Gee K, et al. Cyclosporin A and FK506 inhibit IL-12p40 production through the calmodulin/calmodulin-dependent protein kinase-activated phosphoinositide 3-kinase in lipopolysaccharide-stimulated human monocytic cells. J Biol Chem. 2007;282:13351–13362
- Weimer R, Mytilineos J, Feustel A, et al. Mycophenolate mofetil-based immunosuppression and cytokine genotypes: effects on monokine secretion and antigen presentation in long-term renal transplant recipients. Transplantation. 2003;75:2090–2099
- Haig DM, McInnes CJ, Hutchison G, Seow HF, Reid HW. Cyclosporin A abrogates the acquired immunity to cutaneous reinfection with the parapoxvirus orf virus. Immunology. 1996;89:524–531
- Tang BM, Craig JC, Eslick GD, Seppelt I, McLean AS. Use of corticosteroids in acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med. 2009;37:1594–1603
- Roquilly A, Mahe PJ, Seguin P, et al. Hydrocortisone therapy for patients with multiple trauma: the randomized controlled HYPOLYTE study. JAMA. 2011;305:1201–1209
- Keh D, Boehnke T, Weber-Cartens S, et al. Immunologic and hemodynamic effects of “low-dose” hydrocortisone in septic shock: a double-blind, randomized, placebo-controlled, crossover study. Am J Respir Crit Care Med. 2003;167:512–520
- Meduri GU, Kanangat S, Bronze M, et al. Effects of methylprednisolone on intracellular bacterial growth. Clin Diagn Lab Immunol. 2001;8:1156–1163
- Kaufmann I, Briegel J, Schliephake F, et al. Stress doses of hydrocortisone in septic shock: beneficial effects on opsonization-dependent neutrophil functions. Intensive Care Med. 2008;34:344–349