Abstract
Astragaloside IV (ASI) in Radix Astragali is believed to be the active component. The study aims to investigate whether ASI inhibits tubular epithelial cells apoptosis induced by high glucose and its mechanisms. Tubular epithelial cells in this paper were isolated from human kidney. The cells apoptosis was detected by TUNEL and caspase 3 assay. The protein levels of HGF and TGF-β1 were measured by ELISA. The phospho-p38 production, ERK and JNK were determined by Western blot. ASI could inhibit cells apoptosis induced by high glucose (25 mmol/L) in dose-dependent and time-dependent manners. ASI also inhibited high glucose-induced expression of TGF-β1 and activation of p38 MAPK pathway at the protein level. Furthermore, ASI increased HGF production in human tubular epithelial cells. The ASI inhibition of tubular epithelial cells apoptosis and reduction of TGF-β1 expression induced by high glucose may represent a new treatment for diabetic kidney injury. The mechanism underlying this inhibitory effect may be related to the inhibition of p38 MAPK signaling pathway activation and HGF overproduction.
Introduction
Diabetes is the leading cause of end-stage renal disease in the world.Citation1 Glomerular abnormalities are observed in early diabetic nephropathy (DN) and induce albuminuria.Citation2 As with most chronic degenerative kidney diseases, the gradual inexorable decline of renal function at later stages of DN is invariably associated with tubular epithelial degeneration.Citation3 Recent studies showed that tubular injury and apoptosis were correlated with the severity of DN.Citation2 In our previous study, we demonstrated tubular epithelial cells apoptosis plays an important role in the remodeling process.Citation4 Hepatocyte growth factor (HGF) has multiple effects on renal tubular epithelial cells, HGF can directly reduce tubular epithelial cells apoptosis, and HGF is a potent antifibrogenic factor that can decrease transform growth factor-β1 (TGF-β1) production and collagen biosynthesis.
Astragaloside IV (ASI) is extracted from Radix Astragali, which has long been used in traditional Chinese medicine.Citation5,Citation6 In our previous study, we found that ASI exerts protective effects in diabetic rats through inhibiting TGF-β1 and βig-h3 activities.Citation7 We also found that ASI inhibits TGF-β1 in mesangial cells by stimulating collagen IV production.Citation8 However, whether ASI prevents high glucose-induced kidney tubular injury was not investigated. Thus, we performed experiments to examine the inhibitory effects of ASI on cell apoptosis in tubular epithelial cells induced by high glucose and possible mechanisms of anti-kidney injury activity induced by this compound.
Materials and methods
Preparation and characterization of human epithelial cells cultures
Human kidneys utilized for establishing explant cultures were obtained from kidneys removed at surgery for renal cell carcinoma. The culture of kidney proximal tubule cells was generated using established methods.Citation9 When cells reached confluence, they were passaged by trypsinization, resuspended and replated. These cells showed the specific characteristics of proximal tubular epithelial cells on immunohistochemical examination and morphological observations. Proximal tubular epithelial cells tested positive for cytokeratin, vimentin, alkaline phosphatase, but negative for expression of α-actin, fibronectin and desmin (data not shown).
In vitro experimental design
The cells were seeded in 6-well tissue culture plates. After reaching confluence, the medium was replaced with fresh DMEM/F12 (2% FCS) overnight as all of the cells were in quiescence processes. Where d-glucose was added into the medium then the concentration of the high glucose group was 25 mmol/L glucose. Cells were collected at different time course, respectively.
The ASI were isolated and purified by the Shanghai FuDa Pharmacy, China. ASI were added to the media, the concentration of the media were 25, 50, 100 and 200 μg/mL for 48 h in high glucose for dose-dependent response of ASI, but for time-course study, the cells were stimulated for various time in 100 μg/mL ASI with high glucose. The control group was administered the same volume DMEM/F12.
Caspase 3 assay
This assay was performed in a 6-well plate. For the 6-well plates, 1 × 106 cells were seeded and maintained overnight in DMEM/F12 with 10% FCS. The following day, the old medium was replaced with fresh medium (2% FCS), and the cells were incubated overnight at 37 °C. Following starvation, the cells were stimulated. Following induction, the supernatant cells were centrifuged and collected. The wells were trypsinized to collect the attached cells and combined with the supernatant cells. Caspase 3 activity was performed using the Caspase 3 Fluorometric Assay following the manufacturer’s protocol (R&D systems, Minneapolis, MN). Briefly, cell lysate were incubated on ice, and then added Caspase 3 fluorogenic substrate (DEVD-AFC). Then the cell lysate were incubated at 37 °C for 1 h, read the fluorescent that allow light excitation at 400 nm wave length and can collect emitted light at 505 nm wavelength.
Microscopic detection of TUNEL assay
Cells were seeded at the density of 5000 cells/well on chamber slides and grown in 0.4 mL of DMEM/F12 with 10% FCS. After 2 days, the old medium was aspirated and fresh DMEM with 2% FCS was added, and the cells were starved overnight. The following day new medium was added. Following induction, the slides were washed twice with PBS, and subsequently fixed in fresh 4% formaldehyde/PBS at 4 °C for 25 min. The slides were washed in PBS and the cells permeabilized in 0.2% Triton X-100/PBS for 5 min on ice, then washed with fresh PBS twice for 5 min each at room temperature, and the TUNEL assay was performed as described below.
The TUNEL assay was performed as described in the DNA fragmentation assay kit user manual (Roche, Indianapolis, IN). After the assay, a drop of anti-fade solution was added, and the treated portion of the slide was covered with a glass coverslip with the edges sealed with clear nail polish. Slides were viewed immediately under a fluorescent microscope using filters that allow light excitation at 488 nm wavelength. The images were captured using a digital microscope. For all samples, 15 random fields were chosen, and the number of green cells per field was counted.
Cell viability assay
The tubular epithelial cells were seeded at a density of 5 × 103 cells/well in 96-well plates and cultured for 24 h. Then the cells were treated with various concentrations of ASI (0, 25, 50, 100 and 200 μg/mL). After 24 h, cell viability was measured using the Cell Counting Kit-8 (Dojindo, Japan). Ten microliters of the CCK-8 solution was added into each well of the plate. The cells were incubated for 2 h in the incubator (37 °C and 5% CO2). The absorbance was measured at 450 nm using a microplate reader. Cell viability in each well was presented as a percentage of the control.
Protein HGF and TGF-β1 secretion
The determination of HGF protein levels and TGF-β1 protein were tested by ELISA (purchased from R&D systems). The kit utilizes a sandwich method consisting of three steps of antigen–antibody reactions. Absorbance was read at 450 nm with an automatic microplate reader.
Western-blot analysis
Cells were lysed and lysates were prepared as previously described.Citation10 Protein concentration was determined using Bradford assay. Samples were heated and separated on 10% SDS–PAGE gel. The proteins were electro transferred to a PVDF membrane. Non-specific binding to the membrane was blocked with 2% milk and then the membrane was incubated at 4 °C overnight with specific antihuman phospho-p38, phospho-ERK and phospho-JNK (Santa Cruz Biotechnology, Dallas, TX) antibodies. The signals were visualized by Image J system (Bethesda, MD).
Statistical analyses
Data are reported as mean and SEM for continuous variables. All cell culture experiments were performed at least three times and summarized. Student’s t test and one-way ANOVA analysis were used to determine a significant difference between two groups. All error bars = SD. Statistical significances were considered at p values <0.05.
Results
Astragaloside IV inhibits the cell apoptosis in tubular epithelial cells induced by high glucose concentrations
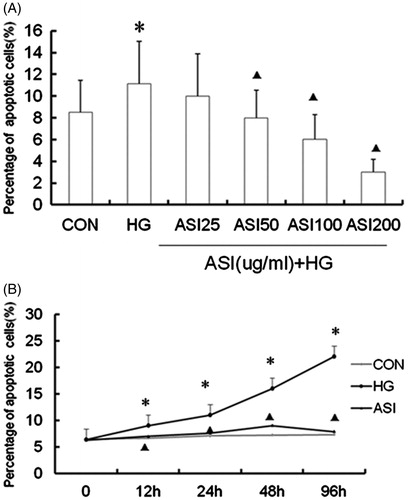
We investigated whether ASI inhibited the cell apoptosis in tubular epithelial cells under high glucose conditions. Cells were treated with increasing concentrations of ASI for 48 h in high glucose and we also stimulated cells in ASI 100 μg/mL with high glucose for various lengths of time. The cell apoptosis was measured using TUNEL assay. As shown in , treatment with high glucose (25 mmol/L) significantly increased cell apoptosis (p < 0.05 compared to normal glucose treatment). However, ASI inhibited high glucose-induced cell apoptosis in a dose-dependent manner, with a maximal inhibitory effect achieved at 200 μg/mL. The significant inhibition caused by ASI was observed within 12 h after the start of pretreatment and increased in a time-dependent manner ().
Figure 1. Effect of Astragalosides IV (ASI) on high glucose-induced apoptosis in tubular epithelial cells. (A) ASI inhibited apoptosis in a concentration-dependent manner. (B) Time course of TUNEL fluorescence in different group. Data are expressed as the mean ± SEM of three independent experiments. *p < 0.05, compared with control group (CON). ▴p < 0.05, compared with high glucose group (HG).
Astragaloside IV can get the balance of HGF and TGF-β1 in tubular epithelial cells under high glucose concentration
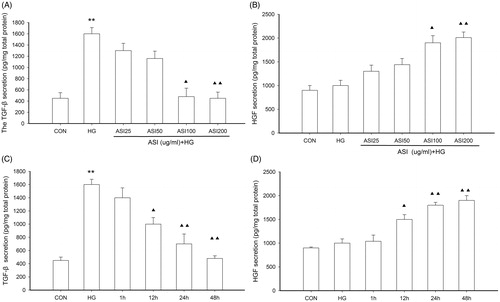
In order to further clarify, the mechanism responsible for the changes in cell apoptosis, we next examined the effect of ASI on HGF and TGF-β1 concentration in cells under high glucose conditions. Tubular epithelial cells were pretreated with increasing concentrations of ASI for 48 h and with various lengths of time in high glucose, the expression of HGF and TGF-β1 in the cells was evaluated by ELISA. As shown in , high glucose significantly increased expression of TGF-β1 (p < 0.05 compared to normal glucose treatment). However, ASI inhibited high glucose-induced secretion of TGF-β1 and increased the expression of HGF in a dose-dependent manner, with a maximal effect achieved at 200 μg/mL (). The inhibition caused by ASI was observed within 12 h after the start of pretreatment and increased in a time-dependent manner (), which showed that ASI could induce a reduction of TGF-β1 activity and supplementation of HGF at the protein level. The results indicated that ASI could influence the balance between TGF-β1 and HGF, which would be effective in ameliorating renal fibrosis.
Figure 2. Effect of ASI on expression of HGF and TGF-β1 in tubular epithelial cells. (A) ASI inhibited the secretion of TGF-β1 protein in concentration-dependent manner. (B) ASI promoted the secretion of HGF protein in concentration-dependent manner. (C) ASI inhibited the secretion of TGF-β1 protein in time-dependent manner. (D) ASI promoted the secretion of HGF protein in time-dependent manner. Data are expressed as the mean ± SEM of three independent experiments. ▴p < 0.05, ▴▴p < 0.01 compared with high glucose groups (HG).
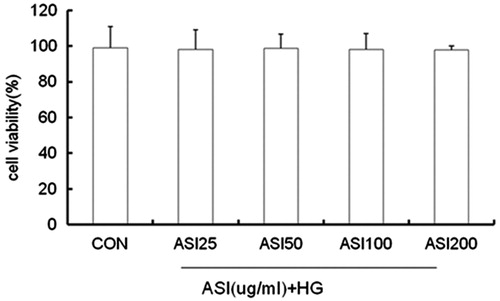
Effect of Astragaloside IV on tubular epithelial cells viability
We next examined the effect of ASI on cell viability to determine whether the inhibitory effect of ASI on expression of TGF-β1 is related to the cytotoxicity induced by this compound. Cells were treated with ASI at concentrations of 25, 50, 100, 200 μg/mL for 24 h. The results showed that ASI at the above concentrations had no inhibitory effect on cell viability ().
Astragaloside IV inhibits high glucose-induced MAPK signaling pathway activation
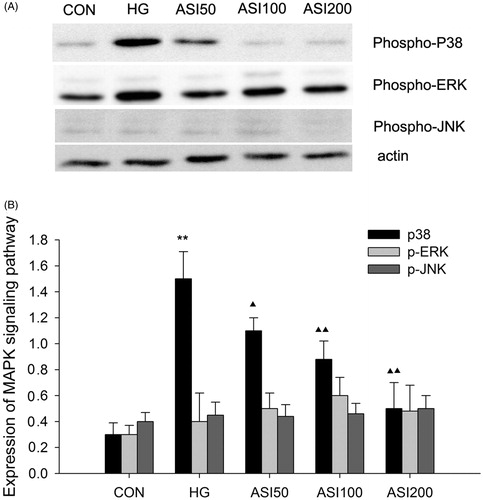
In this study, we explored the effect of ASI on high glucose-induced MAPK signaling pathway activation using Western blotting, β-actin is used for internal control. As shown in , high glucose significantly increased phosphop38 expression by about 5-fold in protein extracts compared to that induced by normal glucose. In contrast, pretreatment with 50, 100 or 200 μg/mL ASI for 48 h markedly inhibited high glucose-induced phosphop38 expression in a dose-dependent manner (). Furthermore, we observed the protein expression of phosphoERK and phospho JNK, the other signaling pathway of MAPK, we cannot find the difference (). On the other hand, we also tested the level of total ERK and JNK, no difference as founded (data not shown).
Figure 4. Effect of ASI on MAPK signaling pathway activation. (A) Bands of phospho-p38, phospho-ERK and phospho-JNK for the indicated concentration of ASI. (B) Semi-quantitative analysis of proteins showed that ASI inhibited the expression of phospho-p38 in concentration-dependent manner, but had no effect on the phospho-ERK and phospho-JNK. Each treatment group is triplicate. Data are expressed as the mean ± SEM. ▴p < 0.05, ▴▴p < 0.01 compared with high glucose groups (HG).
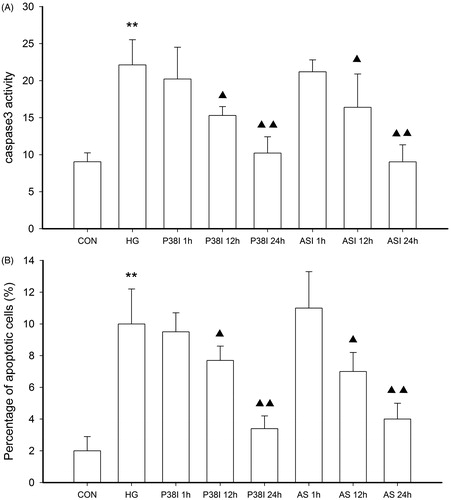
Furthermore, we compared the inhibition ratio of ASI and p38 inhibitor, SB202190, on cell apoptosis in tubular epithelial cells under high glucose conditions (). The cells were pretreated with 100 μg/mL ASI or 1.5 μmol/L SB202190 for 1, 12 or 24 h and then incubated with 25 mmol/L glucose 48 h. The cell apoptosis was analyzed by TUNEL and caspase 3 activity, respectively. As shown in , treatment with ASI and SB202190 for 12 or 24 h could reverse the high glucose-induced cell apoptosis.
Figure 5. The inhibitory effect of ASI and phospho-p38 inhibitor SB202190 on cell apoptosis in tubular epithelial cells under high glucose conditions. The cell apoptosis were then analyzed by caspase 3 activity (A) and TUNEL (B). Data are expressed as the mean ± SEM of three independent experiments. **p < 0.01 compared with control group (CON), ▴p < 0.05, ▴▴p < 0.01 compared with high glucose groups (HG).
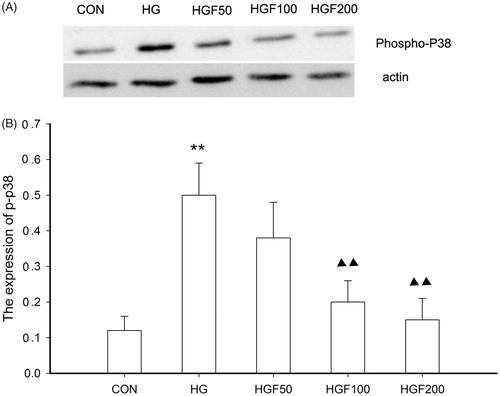
Furthermore, we observed that p38 activation induced by high glucose can be inhibited by HGF (). Our study also showed that HGF had the inhibition of high glucose-induced p38 activation in dose-dependent manner (). In our study, p38 pathway inhibition could also be rapidly induced by HGF pretreatment for 24 h. It showed that p38 MAPK pathway involved the high glucose-induced apoptosis and this process may have relationship with HGF activity.
Figure 6. Effect of HGF on the expression of phospho-p38. (A) Bands of phospho-p38 for the different concentration of HGF. (B) Semi-quantitative analysis of proteins showed that HGF inhibited the expression of phospho-p38 in concentration-dependent manner. Data are expressed as the mean ± SEM of three independent experiments. **p < 0.01, compared with control group (CON). ▴p < 0.05, ▴▴p < 0.01 compared with high glucose groups (HG).
Discussion
The main founding of our study is that, ASI inhibits cell apoptosis, as well as expression of TGF-β1 and the phospho-P38 MAPK signal pathway activation in tubular epithelial cells under high glucose conditions. On the other hand, ASI promoted the production of HGF that can also inhibit cell apoptosis. To our knowledge, this is the first report of an inhibitory effect of ASI on high glucose-induced cell apoptosis and kidney injury.
In our study, incubation of tubular epithelial cells with high glucose significantly induced apoptosis and the expression of TGF-β1. The results were consistent with those of previous studies.Citation11 Accordingly, TGF-β1 is regarded as potential targets for therapeutic intervention in the process of kidney fibrosis.Citation12 Some agents that can interfere with TGF-β1 expression in kidney cells have shown to be effective in treating diabetic kidney fibrosis.Citation13 In our previous study, we found astragalus membranous inhibits TGF-β1 protein and mRNA over expression in renal cortex of diabetic rats suggesting that ASI might play a beneficial role in experimental DN, at least, partly by inhibiting TGF-β1 expression in kidney.Citation7 We also found astragalus membranous can exert a renal protection on DN, possibly partly through the reduction of TGF-β1 and βig-h3 expression.Citation14
In this study, our findings suggest that ASI may represent a novel agent for blocking activity of TGF-β1 and decreasing the cell apoptosis. Our study also showed that high glucose treatment induced HGF degradation. In contrast, pretreatment with ASI prevented HGF degradation and TGF-β1 translocation in a dose-dependent manner, which in turn attenuated cell apoptosis and improved epithelial function. Several cytokines has played an important role on tubulointerstitial fibrosis. Using cultures systems of diabetes, our previous study has shown the expression of HGF is increased in tubular epithelial cells during the early time in the high glucose concentration.Citation4 The activation of HGF expression takes place within 12 h following the high glucose culture. After 24 h, the expression of HGF is suppressed, thus the expression of TGF-β1 is increased. That high glucose can induce the expression of steady-state mRNA levels for TGF-β1, thus leads to ECM accumulation and interstitium fibrosis.
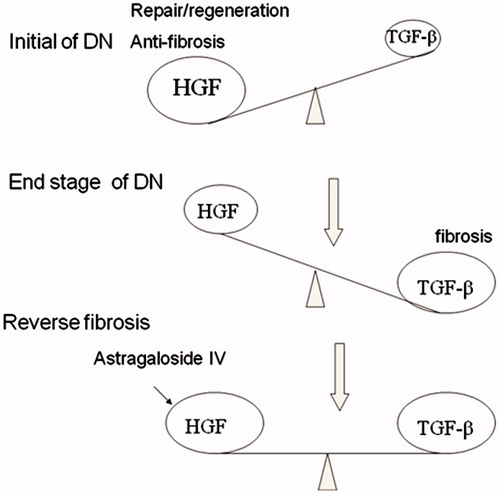
Both HGF and TGF-β1 are initially induced after tissue injury, insinuating that both are necessary and important for the initial wound-healing response and tissue repair.Citation15–17 If the injurious stimulus is transient, such as in the situation after an acute insult, HGF signaling will predominate and prevail, resulting in tissue repair and regeneration, perhaps leading to complete recovery. On the other hand, if the injury is chronic in nature, such as consistent high glucose, TGF-β1 expression progressively increases throughout the entire course of injury, whereas HGF levels initially increase but gradually decline, possibly due to inhibition by TGF-β1. Thus, the net effect after chronic injury is to shift the balance between TGF-β1 and HGF, favoring the profibrotic TGF-β1. In a broad sense, HGF and TGF-β1 function as the Yin and Yang of the tissue fibrotic signals that elicit opposite actions. Therefore, disturbance of the delicate balance between HGF and TGF-β1 activities will eventually cause disastrous consequences ().Citation18 It is likely that ASI to influence the balance between TGF-β1 and HGF would be effective in ameliorating renal fibrosis.
Figure 7. Therapeutic strategies for renal fibrosis. The duration of injury may determine whether the damaged tissues undergo recovery or fibrogenesis. The injury leads to a TGF-β1/HGF ratio that favors HGF, resulting in tissue repair and regeneration, whereas chronic injury dramatically changes the TGF-β1/ HGF ratio to favor TGF-β1, leading to tissue fibrosis. In the fibrotic kidney, the TGF-β1/HGF ratio is out of balance, and TGF-β1 signaling dominates. Thus, therapeutic strategies should include a reduction of TGF-β1 activity and/or supplementation of HGF. It is likely that ASI to influence the balance between TGF-β1 and HGF would be effective in ameliorating renal fibrosis.
In this study, we were interested in the determining the mechanism by which ASI inhibits cell apoptosis in the presence of high glucose. Accumulated evidence has demonstrated that epithelial cell damage induced by high glucose can alter cellular functions via activation of extracellular regulated protein kinase (ERK), C-jun N-terminal kinase (JNK) and p38, the main members of mitogen-activated protein kinase (MAPK) family.Citation19 More recently, Liu et al.Citation19 reported that geniposide another Chinese traditional medicine inhibits cell apoptosis in endothelial cells by blocking p38 and ERK1/2 signaling pathways. Therefore, ERK, JNK and p38, may also be involved in high glucose-induced apoptosis. We hypothesized that inhibition of cell apoptosis by ASI may be related to inhibition of the MAPK signal pathway. In our study, exposure of epithelial cells to high glucose resulted in increased levels of phospho-P38. However, this overproduction was prevented by pretreatment with 50–200 μg/mL ASI for 24 h. We first investigated ASI inhibited high glucose-induced MAPK signal pathway overproduction. At the same time the phospho-p38 inhibitor SB202190 can also inhibit the cell apoptosis. Our results suggest that the inhibitory effect of ASI on cell apoptosis may be related to inhibition of the p38 MAPK signal pathway, but not ERK1/2 and JNK. We investigated that HGF can inhibit high glucose induced phosphop38 expression. The results suggested that the inhibitory effects of ASI on cell apoptosis might be related to its anti-phospho-P38 MAPK signal activity and the balance of TGF-β1 and HGF activity. Taken together, the p38 pathway may be involved in the inhibitory effect of ASI on high glucose-induced cell apoptosis and the balance of HGF and TGF-β1. However, there was discrepancy in ASI function on cell apoptosis. Yuan et al.Citation20 previously reported that ASI promotes apoptosis in rat vascular smooth muscle cells under high glucose concentration in vitro.Citation20 But Yuan’s work focused on rat vascular smooth muscle cells, our experiment adopted human kidney epithelial cells. Different cell lines may receive different response. Previous studies also showed ASI could inhibit PC12 cells apoptosis induced by oxidative damage.Citation21 Consequently, our work may provide more clues for ASI function research.
In conclusion, the present study demonstrates that ASI inhibits high glucose-induced cell apoptosis in human tubular epithelial cells. The mechanism underlying the inhibitory effects of this compound on cell apoptosis and TGF-β1 expression may occur through increasing of HGF overproduction and inhibition of MAPK signal pathway activation. Our findings suggested that ASI, a novel cell apoptosis inhibitor, may be used as a preventive agent for diabetic kidney injury.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article. This work was supported in part by National Basic Research Program of China 973 Program No. 2012CB517600 (No. 2012CB517602). The project was also sponsored by the National Natural Science Foundation of China (81102700 and 81373865), National “Twelfth Five-Year” Plan for Science & Technology Support No. 2011BAI10B00 and by grants 10JC1410100 and 12401906400 from the Science and Technology Commission of Shanghai Program ZXSNXD-CC-ZDYJ002 and 2010QL040A from the Shanghai Health Bureau and Funding scheme for training young teachers in Colleges and Universities in Shanghai were also included.
References
- Renal Data System: USRDS 1998 Annual Data Report (NIH Publ. No. 98–3176). Washington, DC: US Government Printing Office; 1998
- Isermann B, Vinnikov IA, Madhusudhan T. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007;11:1349–1358
- Beyenbach KW. Kidneys sans glomeruli. Am J Physiol Renal Physiol. 2004;286:F811–F827
- Mou S, Wang Q, Shi B, et al. Hepatocyte growth factor ameliorates progression of interstitial injuries in tubular epithelial cells. Scand J Urol Nephrol. 2010;44:121–128
- Hei ZQ, Huang HQ, Zhang J, et al. Protective effect of Astragalus membranaceus on intestinal mucosa reperfusion injury after hemorrhagic shock in rats. World J Gastroenterol. 2005;11:4986–4991
- Jia RZ, Jiang L, Qiao LX. Study on effct of radix astragali on injury of cerebral cortex in neonatal rats after hypoxia/ischemia brain damage. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25:54–57
- Ni ZH, Zhang QY, Qian JQ, et al. Effect of Astrgalin on matrix secretion and β1 integrin mRNA expression in human mesangial cells. Chin J Nephrol. 2000;16:303–307
- Xu YJ, Zhang QY, Lu M, et al. Effect of Astragulus membranaceus on expression of TGF-β in renal cortex of diabetic rats. Chin J Endocrinol Metab. 1998;14:312–314
- Sheridan AM. Renal mouse proximal tubular cells are more susceptible than MDCK cells to chemical anoxia. Am J Physiol. 1993;265:F342–F350
- Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142
- Border WA, Noble NA. Interactions of transforming growth factor-β and angiotensin II in renal fibrosis. Hypertension. 1998;31:181–188
- Dai C, Liu Y. Hepatocyte growth factor antagonizes the profibrotic action of TGF-beta1 in mesangial cells by stabilizing Smad transcriptional corepressor TGIF. J Am Soc Nephrol. 2004;15:1402–1412
- Liu ZQ, Li QZ, Qin GJ. Effect of Astragalus injection on platelet function and plasma endothelin in patients with early stage diabetic nephropathy. Chin J Integr Tradit West Med. 2001;21:274–276
- Yang R, Ni ZH, Qian JQ, et al. The significance of βig-h3 expression in renal tissue of diabetic rats and the efects oflosartan and astragalus memberaneous. Chin J Nephrol. 2004;10:347–350
- Liu Y. Hepatocyte growth factor in kidney fibrosis: therapeutic potential and mechanisms of action. Am J Physiol Renal Physiol. 2004;287:F7–F16
- Mizuno S, Nakamura T. Suppressions of chronic glomerular injuries and TGF-β1 production by HGF in attenuation of murine diabetic nephropathy. Am J Physiol Renal Physiol. 2004;55:F134–F143
- Taniyama Y, Morishita R, Nakagami H, et al. Potential contribution of a novel antifibrotic factor, hepatocyte growth factor, to prevention of myocardial fibrosis by angiotensin II blockade in cardiomyopathic hamsters. Circulation. 2000;102:246–252
- Coltella N, Rasola A, Nano E. p38 MAPK turns hepatocyte growth factor to a death signal that commits ovarian cancer cells to chemotherapy-induced apoptosis. Int J Cancer. 2006;118:2981–2990
- Liu HT, He JL, Li WM, et al. Geniposide inhibits interleukin-6 and interleukin-8 production in lipopolysaccharide-induced human umbilical vein endothelial cells by blocking p38 and ERK1/2 signaling pathways. Inflamm Res. 2010;59:451–461
- Yuan W, Zhang Y, Ge Y, et al. Astragaloside IV inhibits proliferation and promotes apoptosis in rat vascular smooth muscle cells under high glucose concentration in vitro. Planta Med. 2008;74:1259–1264
- Huang XP, Liu XD, Deng CQ. Effects of the combination of active component extracts from Astragalus membranaceus and Panax notoginseng on apoptosis, reactive oxygen species and mitochondrial membrane potential of PC12 cells with oxidative injury. J Chin Integr Med. 2012;10:1127--1134