Abstract
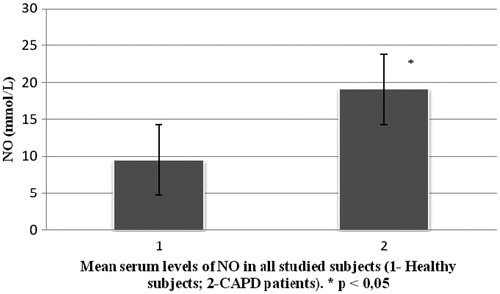
End-stage renal disease (ESRD) and its treatment modules affect almost all organs and organ systems including vascular endothelium. It is well known that disturbance of vasoactive substances (nitric oxide – NO and endothelin-1 – ET-1) production appears in these patients. There is a small number of studies which investigated serum levels of NO and ET-1 in ESRD patients treated with continuous ambulatory peritoneal dialysis (CAPD). Therefore our study aimed to measure serum levels of NO and ET-1 in this population. This study included 23 ESRD patients (10 males and 13 females) treated with CAPD, mean age 55.8 ± 15.8 years. Mean duration of CAPD treatment in this group of patients was 3.4 ± 14.7 years. Besides this group of patients (CAPD), we included a second group which consisted of 30 healthy controls [14 males, 16 females, mean age 51.8 (±15.6) years]. Our results show significantly higher serum levels of NO in CAPD patients ( ± SD = 19.09 ± 6.9) in comparison to the control group (
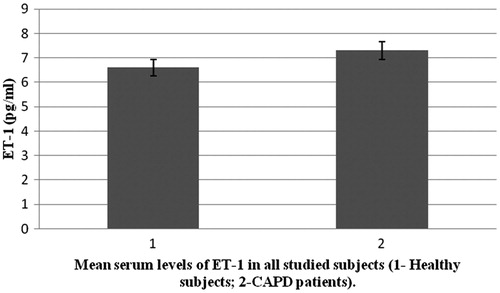
± SD = 9.5 ± 1.9) (p < 0.05). There was no significant difference in serum levels of ET-1 between CAPD patients (
± SD = 7.3 ± 5.6) and the control group (
± SD = 6.6 ± 4.2), (p > 0.05). From our results, we concluded that imbalance in production of vasoactive substances is present in CAPD patients. This imbalance can lead to disturbance in local blood flow control. These pathophysiological mechanisms can cause significant hemodynamic disturbance (hypertension) and atherosclerosis.
Introduction
Vascular endothelium is not just a mechanical barrier in blood vessel, but endocrine organ as well which produces many substances out of which some have vasoactive effects. Two most potent vasoactive substances with opposite effects are nitric oxide (NO) vasodilatator and endothelin-1 (ET-1) one of the most powerful known vasoconstrictor.Citation1,Citation2 Besides its vasoactive effects, these substances (NO and ET-1) express many others metabolic and biochemical effects.Citation3,Citation4 End-stage renal disease (ESRD) requires treatment with one of dialysis models. Physiological and pathophysiological mechanisms in these patients very often can cause damage of endothelium or blood vessel in whole. All these disorders in blood vessels can cause endothelial dysfunction.Citation5–7 It is well known that cardiovascular diseases (e.g., hypertension) are leading cause of death in ESRD patients. Endothelial damaging as well as imbalance in production of vasoactive substances can be connected with these facts.Citation8–11 Moreover, all dialysis modules attribute to this cardiovascular and endothelial damaging.
There is a small number of studies which investigated serum levels of NO and ET-1 in ESRD patients treated with continuous ambulatory peritoneal dialysis (CAPD). Therefore, our study aimed to measure serum levels of NO and ET-1 in this population.Citation6,Citation12
Material and methods
We performed a prospective study which included ESRD patients treated with CAPD. The CAPD group included 23 patients (10 males, 13 females, mean age 55.8 ± 15.8 years) who were treated with CAPD at the Institute for nephrology of University hospital in Niš. Dialysis solution was changed three times per day and patients were trained to do it by themselves or it was done at the Institute under the supervision of the medical staff.
Beside CAPD group of patients, we included a second group which consisted of 30 healthy subjects (14 males, 16 females, mean age 51.8 ± 15.6 years) to serve as a control group. We measured the levels of NO and ET-1 in the second group and its mean level (±SD) served as a referent value. All studied subjects where non smokers. represents basic demographic characteristics of patients and control group. shows CAPD patients regarding primary diseases which caused ESRD.
Table 1. Basic demographic characteristics of patients.
Table 2. CAPD patients regarding primary disease.
Blood samples from all observed patients were taken from cubital vein.
From the patients in CAPD group (patients treated with CAPD), blood samples were collected immediately before emptying of peritoneal cavity. From the control group, blood were collected in basal conditions.
Measurement of NO serum levels
The NO level in whole blood is determined by measuring nitrite and nitrate ( and
) production using classical colorimetric reaction (Griess). Blood samples for the determination of NO concentration were diluted 1:1 (vol/vol) with 0.9% saline, protein-precipitated using 30% ZnSO4, 0.05 mL per millilitre of blood and centrifuged at 700 g for 10 min and frozen at −20 °C. Conversion of
into
was done with nitrate reductase elementary zinc.
concentration in serum was determined by classic colorimetric Griess reaction. Briefly, equal volumes of samples and Griess reagent (sulfanilamide and naphthalene–ethylene diamine dihydrochloride) were mixed at room temperature. After 5 min, the absorbance was measured at 546 nm using spectrophotometer. The concentration of nitrite was determined by a standard curve prepared with sodium nitrite.
Measurement of ET-1 serum levels
From the whole blood specimen, serum was separated in Heated bath on 37 °C. Activity of serum ET-1 was measured with EIA methodology which is based on immunometric assay so called sandwich technique. Measurement was performed using computer based ELISA reader (ELx 800 Universal Microplate Reader Biotek Instruments, Inc) with wavelength 405 nm. We used prepared enzyme kit (ET-1; EIA kit – IBL Hamburg, Germany). Endothelin test kit used in this study was an enzyme radioimmunoassay designed for direct determination of ET-1 in biological fluids.
Statistical analysis
The results were processed using standard statistical method (Student’s t-test) for small independent samples (modification by Cochran & Cox) shown as mean ± standard mean error ( ± SX). We tested significance of the difference in mean values between studied groups with an aim to monitor changes in serum NO levels. We considered the value of p < 0.05 statistically significant.
Results
Study included 23 CAPD patients and 30 patients in the control group. Demographic characteristics of all 53 patients are shown in . shows CAPD patients regarding primary diseases which caused ESRD. presents comparison of mean serum levels of NO in CAPD group of patients (19.09 ± 6.9) and control group (9.5 ± 1.9). Statistical analysis with Student’s t-test for small independent samples (Cochran and Cox modification) showed significantly higher serum levels of NO in CAPD patients in comparison to control group (p < 0.05). presents comparison of mean serum levels of ET-1 in CAPD group of patients (7.3 ± 5.6) and control group (6.6 ± 4.2). Statistical analysis with Student’s t-test for small independent samples (Cochran and Cox modification) demonstrated higher serum levels of ET-1 in CAPD patients in comparison to control group but without statistical significance (p > 0.05).
Discussion and conclusion
Our study shows significantly higher serum levels of NO in patients treated with CAPD compared to control group (). Some other authors and literature data regarding physiology and pathophysiology of NO in ESRD patients treated with one of dialysis modes are contradictory.Citation6 One group of data suggests that serum levels of NO in ESRD patients treated with some mode of dialysis are significantly lower in comparison to healthy subjects, while other states there no statistically significant difference in NO serum levels between these observed groups.Citation13–15 One possible explanation for lower serum levels of NO in uremic patients could be decreased concentration of l-arginine due to reduction of renal parenchyma. Besides this, some authors found increased levels of endogen inhibitors of NO synthase (NOS) such as asymmetric dimethylarginine (ADMA). This overload of ADMA is presented in dialyzed patients as well.Citation16–18 Reduction in renal mass leads to increase in secretion of proinflammatory mediators platelet-derived growth factor and transforming growth factor. Both of these mediators are very potent inhibitors of NOS.
Recent in vitro and in vivo data on rats have shown that a decreased renal mass leads to an increased synthesis of a potent vasoconstrictor, ET-1, which decreases the production of NO.Citation14,Citation19,Citation20 In our study, serum levels of NO were significantly higher in CAPD patients compared to healthy population (p < 0.05) (). Explanation for these results can be found in increase production of NO from mesothelial cell. Davenport et al.Citation21,Citation22 state that mesothelial and endothelial cell originate from the same germ layers. Besides this way of NO production, tissue macrophages which are involved in inflammatory processes in peritonitis represent significant source of NO.Citation23 This is likely because CAPD patients develop bacterial peritonitis frequently.Citation24 By investigating physiology and pathophysiology of these changes in CAPD patients, Imai et al.Citation25 found that patients with chronic hypotension compared to normotensive patients have similar stroke volumes and heart rates, but lower levels of peripheral vascular resistance. There is an assumption that some ESRD patients during progression of their disease start to produce vasodilators intensively, primarily NO and adrenomedullin. Noris et al.Citation23 suggest that thrombocytes can also significantly contribute to NO production. We also found serum levels of ET-1 to be higher in CAPD patients compared to control group, although statistical significance was not reached (). The literature regarding physiology and pathophysiology of ET-1 in ESRD patients treated with one of dialysis modes provides conflicting data, although most authors state that ET-1 serum levels are higher in these patients compared to healthy subjects.Citation12
In the glomeruli affected by sclerosis, endothelial injury leads to the increased secretion of ET-1 and consequent vasoconstriction, increased intraglomerular pressure, and decreased glomerular filtration for which it is suspected to be one of main reasons for elevated serum ET-1 levels.Citation20,Citation26 On the other hand, slight increase ET-1 in peritoneal cavity and application of human recombinant erythropoietin can contribute to increase serum levels of ET-1. Besides increased production of ET-1, Lebel et al. found lower elimination of ET-1 via peritoneal membrane.Citation27–31
From our results, we concluded that imbalance in production of vasoactive substances (NO and ET-1) is present in CAPD patients. This imbalance can lead to disturbance in local blood flow control. These pathophysiological mechanisms can cause significant hemodynamic disturbance that may lead to hypertension and atherosclerosis.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Furchgott RF, Zawadski JV. The obligatory role of endothelial cell in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376
- Hickey KA, Rubanyi GM, Paul RJ, Higsmith RF. Characterization of a coronary vasoconstrictor produced by endothelial cells in culture. Am J Physiol. 1985;248:500–556
- Hirst DG, Robson T. Nitric oxide physiology and pathology. Methods Mol Biol. 2011;704:1–13
- Derentowicz P, Markiewicz K, Wawrzyniak M, Czerwińska-Kartowicz I, Buława E, Siwińska-Gołebiowska H. Nitric oxide (NO) – Nobel prize in medicine and physiology for 1998. Med Wieku Rozwoj. 2000;4:209–217
- Carracedo J, Buendía P, Merino A, et al. Cellular senescence determines endothelial cell damage induced by uremia. Exp Gerontol. 2013;48:766–773
- Meenakshi SR, Agarwal R. Nitric oxide levels in patients with chronic renal disease. J Clin Diagn Res. 2013;7:1288–1290
- Brunini TM, Moss MB, Siqueira MA, Santos SF, Lugon JR, Mendes-Ribeiro AC. Nitric oxide, malnutrition and chronic renal failure. Cardiovasc Hematol Agents Med Chem. 2007;5:155–161
- de Leeuw PW. Pathophysiology of hypertension in patients in renal replacement therapy. Blood Purif. 1994;12:245–251
- Davies DL, Schalekamp MA, Beevers DG, et al. Abnormal relation between exchangeable sodium and the renin-angiotensin system in malignant hypertension and in hypertension with chronic renal failure. Lancet. 1973;1:683–686
- Campigano JL, Ramirez-Muzo O, Ramirez-Gonzales R. Normal renin uremic hypertension: study of cardiac hemodynamics, plasma volume, extracellular fluid volume, and the renin-angiotensin system. Arch Intern Med. 1976;136:17–23
- Tuckman J, Benninger JL, Reubi F. Hemodynamics and blood volume studies in long-term hemodialysis patients and in patients with successfully transplanted kidneys. Clin Sci Mod Med. 1973;45:155–157
- Lightfoot BO, Caruana RJ. Endothelin-1 in continuous ambulatory peritoneal dialysis and hemodialysis patients: a preliminary study. Perit Dial Int. 1993;13:55–58
- Reyes AA, Karl IE, Klahr S. Role of arginine in health and in renal disease. Am J Physiol. 1994;267:331–346
- Leone A, Moncada S, Vallance P, Calver A, Collier J. Acumulation of an endogenus inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575
- Schmidt RJ, Baylis C. Total nitric oxide production is low in patients with chronic renal disease. Kidney Int. 2000;58:1261–1266
- Hand MF, Haynes WG, Webb DJ. Hemodialysis and L arginin but not D arginin correct renal faliure associated endothelial dysfunction. Kidney Int. 1998;53:1068–1077
- Kari JA, Donald AE, Vallance DT, et al. Physiology and biochemistry of endothelial function in children with chronic renal failure. Kidney Int. 1997;52:468–472
- Morris ST, McMurray J, Rodger R, Jardine AG. Impaired endothelinum-dependent vazodilatation in uremia. Nephron Dial Transplant. 2000;15:572–575
- Madore F, Prudhomme L, Austin JS, et al. Impact nitric oxide in blood pressure in hemodialysis patients. Am Kidney Dis. 1997;30:665–671
- Potter GS, Johnson RJ, Fink GD. Role of endothelin in hypertension of experimental chronic renal failure. Hypertension. 1997;30:1578–1584
- Devenport A, Fernando RL, Varghese Z. Intraperitoneal nitric oxide production in patients treated by continuous ambulatory peritoneal dialysis. Blood Purif. 2004;22:216–223
- Davenport A, Fernando RL, Robson R, Varghese Z. Nitric oxide production by human peritoneal mesothelial cells. Int J Artif Organs. 2004;27:15–23
- Noris M, Benigni A, Boccardo P, et al. Enhanced nitric oxide synthesis in uremia: implications for platelet dysfunction and dialysis hypotension. Kidney Int. 1993;44:445–450
- Su YJ, Liao SC, Cheng BC, Hwang JC, Chen JB. Increasing high-sensitive C-reactive protein level predicts peritonitis risk in chronic peritoneal dialysis patients. BMC Nephrol. 2013;14:185
- Imai Y, Abe K, Otsuka Y, et al. Blood pressure regulation in chronic hypotensive and hypertensive patients with chronic renal failure. Jap Circ J. 1981;45:303–314
- Bussemaker E, Passauer J, Reimann D, Schulze B, Reichel W, Gross P. The vascular endothelin system is not overactive in normotensive hemodialysis patients. Kidney Int. 2002;62:940–948
- Kang DH, Yoon KI, Han DS. Acute effects of recombinant human erythropoietin on plasma levels of proendothelin-1 and endothelin-1 in hemodialysis patients. Nephrol Dial Transplant. 1998;13:2877–2883
- Lebel M, Moreau V, Grose JH, Kingma I, Langlois S. Plasma and peritoneal endothelin levels and blood pressure in CAPD patients with or without erythropoietin replacement therapy. Clin Nephrol. 1998;49:313–318
- Kourti P, Zarogiannis SG, Liakopoulos V, et al. Endothelin-1 acutely reduces the permeability of visceral sheep peritoneum in vitro through both endothelin-A and endothelin-B receptors. Artif Organs. 2013;37:308–312
- Kourti P, Zarogiannis S, Liakopoulos V, et al. Effect of endothelin-1 on the transmesothelial resistance of isolated visceral sheep peritoneum. Adv Perit Dial. 2007;23:38–42
- Morgera S, Kuchinke S, Budde K, Lun A, Hocher B, Neumayer HH. Volume stress-induced peritoneal endothelin-1 release in continuous ambulatory peritoneal dialysis. J Am Soc Nephrol. 1999;10:2585–2590