Abstract
Transplantation tolerance is still a Utopian dream for many transplanters. Mesenchymal stem cells (MSC) have shown immuno-modulatory and tolerogenic effects in experimental models. We present a 29-year-old male with end stage renal disease (ESRD) who was transplanted with HLA 4/6 matched kidney from 51-year-old father in June 2010 preceded by co-infusion of donor-adipose tissue derived mesenchymal stem cells (AD-MSC) and bone marrow derived hematopoietic stem cells (BM-HSC) under non-myeloablative conditioning for deleting rejecting T and B-cells. He has maintained fairly stable graft function with serum creatinine (SCr) between 1.5 and 1.8 mg/dL at 3 years post-transplant with absence of donor specific antibodies (DSA), normal protocol graft biopsy, and peripheral T-regulatory cell levels (pTregs) (CD127low/−CD25highCD4+) of 4.57% on zero immunosuppression since 6 months.
Introduction
Acute rejections can now be controlled after transplantation using induction therapies however chronic graft attrition still remains an unanswered enigma in spite of maintenance immunosuppression. Mesenchymal stem cells (MSC) have shown promising tolerogenic and immunomodulatory role.Citation1,Citation2 We have been using different kinds of stem cells (SC) including donor-adipose tissue derived MSC (AD-MSC) in renal transplant (RT) recipients to achieve minimization of immunosuppression with variable non-myeloablative conditioning regimens.Citation3 This case demonstrates that transplant tolerance can be induced using SC therapy (SCT).
Case report
A 29-year-old male with end stage renal disease (ESRD) due to unknown etiology presented for RT with SCT. He was on maintenance hemodialysis for 2 months and had not received any blood transfusion. He had unremarkable general and systemic examination except blood pressure of 170/100 mmHg at presentation, which was controlled with Nifedipine, 20 mg thrice a day and Atenolol, 50 mg twice a day. His serum creatinine (SCr) was 4.9 mg/dL, hemoglobin, 11.4 gm/dL, total white cell count, 11.9 × 104/µL, platelet count, 2.67 × 105/µL, blood group “O” Rh positive, and he was non-reactive for hepatitis B/C and HIV infections. His anti-nuclear antibodies, anti-cytoplasmic antibodies and anti-cardiolipin antibodies were negative with normal serum C3 and C4 levels. Liver functions, electrolytes, blood sugar, lipid profile, and uric acid were within normal limits. Urine routine examination revealed +3 albumin, microscopy was unremarkable and was sterile on culture sensitivity testing. Echocardiography showed global left ventricular hypokinesia with ejection fraction of 55%. Fundus examination showed grade-3 hypertensive retinopathy. Lymphocyte cross-match using standard CDCC technique (LCM) was negative, single antigen (SA) assay using Luminexx platform showed the absence of antibodies and flow-cross-match with T and B-cells (FCM) [cut-off-50 median channel shift (MCS) for T-cells and 150 MCS for B-cells] were negative.
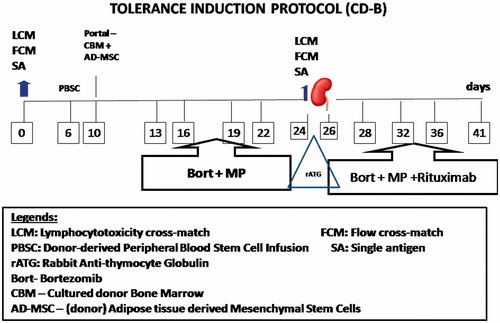
He was subjected to laparoscopic RT after favorable immune response of negative LCM, FCM and SA, with HLA 4/6 match, 51-year-old father’s kidney, blood group “O” positive, in June 2010 under tolerance induction protocol (TIP; ) consisting of peripheral blood stem cells (PBSC; 188 mL) in periphery and combined infusion of AD-MSC (3 mL) and CBM-HSC (99 mL) in portal circulation.
Figure 1. Tolerance Induction Protocol paradigm: On day 0 and 24, immune monitoring was performed by lymphocyte cross-match (LCM), using CDCC cross-match, flow cross-match for T and B-cells and donor specific antibody (DSA) by single antigen assay using Luminexx solid phase assay. Peripheral blood stem cells collected from donor were infused in peripheral circulation of recipient after dialysis, on day 6 and stem cells generated from donor were infused in portal circulation by minilaparotomy. On days 13, 16, 19, and 22 Bortezomib, 1.3 mg/m2 was administered intravenously (IV) along with methylprednisone (MP), 250 mg after favorable immune monitoring with LCM, FCM, and SA. Induction was done by rabbit anti-thymocyte globulin, 1.5 mg/kg BW intraoperatively with MP, 500 mg IV on days −1, 0, +1, 250 mg on day +2 and 125 mg on day +3 after transplantation. Rituximab was administered on third post-operative day followed by Bort on day 28, 32, 36, and 41, respectively, with close monitoring for complete blood counts and platelet counts.
For portal infusion, a midline incision of approximately 3–5 cm length was made above the umbilicus by laparotomy under local anesthesia, omental vein was identified and cannulated with 20 gauze Intracath. Stem cell bag was connected and they were infused directly without using any filters, at the rate of 6–8 mL/min. After infusion, omental vein was ligated with silk and hemostasis was checked. The wound was closed with Vicryl 2/0, and subcuticular stitches were taken using 3/0 Monocryl.
Nucleated cell count of CBM and PBSC was 2.18 × 104/µL and 1.9 × 105/µL, respectively, with CD34+, 1.9 × 106/kgBW in CBM and 0.22 × 106/kgBW in PBSC. The nucleated cell count of AD-MSC was 2.5 × 102/µL with CD45−/90+ 20.74% and CD73+, 12.35%. Non-myeloablative conditioning was with Bortezomib, 1.3 mg/m2 in four doses (day 1, 4, 7, and 11), both before and after RT along with 125 mg intravenous methylprednisone. Induction included methylprednisone, 500 mg, on day before, day 0 and day after transplantation, rabbit-antithymocyte globulin, 1.5 mg/kgBW during anastomosis followed by Rituximab, 375 mg/m2 on third post-operative day. Methylprednisone was tapered to 250 mg on second and 125 mg on fourth post-operative day. Anastomosis time was 60 min. Prednisone, 20 mg/day was given for maintenance immunosuppression.
Immune monitoring consisted of SA at weekly intervals. Complete blood counts including platelet counts, SCr and urine routine examination were monitored twice weekly for first month, weekly for next 2 months, fortnightly for next 3 months and monthly thereafter. He was also monitored for CMV infection at 3 monthly intervals.
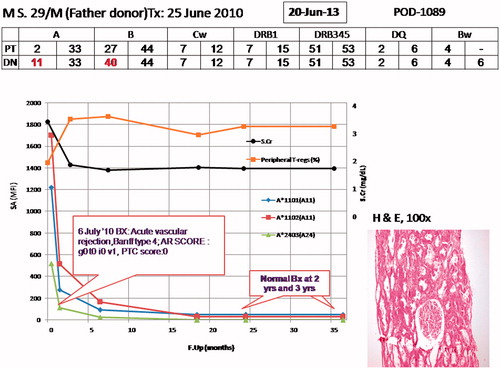
Stem cell infusion, conditioning and RT were uneventful. However he had slow graft function (SGF). On 12th post-operative day, he developed biopsy-proven C4d negative acute vascular rejection, Banff type ag0 at0 av1 ai0, PTC score 0. Hence he was subjected to anti-rejection therapy of methylprednisone, 250 mg × 3, intravenous immunoglobulin, 100 mg/kgBW × 5, and plasmapheresis, five sessions on alternate days with 2 L of plasma exchanged in every session (60% crystalloid, 40% colloid of 20% human albumin). Rescue immunosuppression was started on day 13. This consisted of Tacrolimus, 0.05 mg/kgBW/day and mycophenolate mofetil (MMF), 2 gm in 4 divided doses, and Prednisone was continued at 20 mg/day under prophylaxis for fungal and viral infections. At 2-year post-transplant his protocol graft biopsy was unremarkable with absence of DSA and stable SCr around 1.6 mg/dL. Immunosuppression weaning was started beginning with Tacrolimus. Eventually all immunosuppressants were withdrawn under monitoring of SCr, DSA, and pTregs (CD127low/−CD25highCD4+). He did not have any viral/fungal/bacterial infections. At 3 years post-transplant, he has achieved tolerance with stable graft function on zero immunosuppression since 6 months, pTregs of 4.57%, absence of DSA, and normal graft biopsy ().
Figure 2. Graph showing the complete status of patient; on top is his age, initials, mentioning father as donor, and transplant date. On top in box is the date when this graph was prepared and right side mentions days since induction of protocol. HLA match status of patient (PT) and donor (DN) are mentioned. On the X-axis is the follow-up in months since the patient was inducted in the protocol, on Y-axis left is antibody measurement by Luminexx assay (in mean fluorescent intensity – MFI) and on right side is serum creatinine (SCr; mg/dL) and peripheral T-regulatory cells (CD127low/−CD25highCD4+) in percentage. The different colored spikes are for antibodies shown by asterix in right side, black line is for SCr and yellow line for p-Tregs. On the graph are highlighted biopsy dates and findings. This figure shows that SCr and p-Tergs have remained fairly stable. On right corner is photomicrograph showing renal allograft biopsy performed at 3 years post-transplant showing normal glomerulus, part of a medium calibre artery and surrounding tubules and 1 artery, Hematoxylin and Eosin, 100×.
Discussion
Anecdotal reports of transplant tolerance using HSCs and/or conditioning regimens are seen in literature. All these attempts were made by weaning off immunosuppression.Citation4 As against these attempts, we have attempted transplantation tolerance beginning with zero immunosuppression here.
MSC were helpful in achieving tolerance due to their immunosuppressive and tolerogenic properties in addition to enhancing the action of HSC.Citation1,Citation5,Citation6 Tregs are involved in maintenance of tolerance. MSC have been considered as a potential “homeostatic niche” for Tregs and play role in Treg recruitment, regulation and maintenance in vitro. MSCs migrate preferentially to lymphoid organs and allografts where they interact with the activated immune cells and modulate their function.Citation7 Briefly their function can be mentioned in three principal mechanisms of evading allogeneic rejection, namely being hypoimmunogenic; modulating T cell phenotype by converting naïve T-cells to regulatory T-cells instead of cytotoxic T cells, and finally, by creating an immunosuppressive local milieu.Citation8 These mechanisms are inter-related and involve cell contact dependent and independent interactions.
Tregs play a crucial role in tolerance induction by the mechanisms of infectious tolerance and linked suppression. This pathway has brought in solution to chronic rejection was evading all transplanters so far. The tolerating potential of a single alloantigen, which can subsequently dominantly confer non-responsiveness against all other antigens present within the graft, has been termed “linked suppression” and is dependent on the action of Tregs.Citation9 Linked suppression occurs when a potentially alloreactive T cell comes under the tolerizing influence of a Treg as both cells recognize their respective alloantigens presented by the same APC.Citation10–12 Infectious tolerance can be simply explained as converting other T-cell phenotypes into Tregs themselves. In effect, this leads to a “reeducation” of the potentially destructive alloresponsive T cell with the induction of a Treg phenotype. A reduction in the uniquely high numbers of potentially graft destructive T cells would facilitate immunoregulation by Treg subsets, thus promoting and maintaining a tolerant state.
We could achieve tolerance in our patient for number of reasons, he was immunologically low risk patient, he had good HLA match including DR match, and MSC helped in overcoming immune injury following SGF.
Conclusion
MSC play a crucial role in induction and maintenance of transplantation tolerance in LDRT through generation and recruitment of Tregs.
Author contribution
HLT was the principal investigator of the study and was responsible for patient care and helped in writing the manuscript; AVV carried out the laboratory research work and wrote the manuscript; SGC and AK helped in manuscript writing and literature survey; SDD carried out stem cell lab work.
Declaration of interest
None of the authors have any conflict of interest regarding this manuscript.
References
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822
- Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105(5):2214–2219
- Vanikar AV, Goplani KR, Feroz A, et al. Operational tolerance in living-related renal transplantation: a single-center experience. Transplant Proc. 2011;43(5):1551–1558
- Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and withdrawal of immunosuppressive drugs in patients given kidney and hematopoietic cell transplants. Am J Transplant. 2012;12(5):1133–1145
- Koc ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18(2):307–316
- Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–48
- Shi M, Liu ZW. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011;164:1–8
- Trivedi HL, Vanikar AV. Mesenchymal stem cells and solid organ transplantation. Cell R4. 2013;1(2):123–136
- Waldmann H, Cobbold S. Regulating the immune response to transplants: a role for CD4+ regulatory cells? Immunity. 2001;14:399–406
- Honey K, Cobbold SP, Waldmann H. CD40 ligand blockade induces CD4+ T cell tolerance and linked suppression. J Immunol. 1999;163:4805–4810
- Davies JD, Leong LY, Mellor A, et al. T cell suppression in transplantation tolerance through linked recognition. J Immunol. 1996;156:3602–3607
- Stassen M, Schmitt E, Jonuleit H. Human CD(4+)CD(25+) regulatory T cells and infectious tolerance. Transplantation. 2004;77(1 Suppl):S23–S25
