Abstract
Aim: Long-term survival of renal allografts has improved over the last 20 years. However, less is known about current expectations for long-term allograft function as determined by estimated glomerular filtration rate (eGFR). The aim of this study was to investigate factors which affect graft function at 5 years’ post-renal transplantation. The statistically significant factors were then used to construct a predictive model for expected eGFR at five years’ post-transplant. Methods: We retrospectively reviewed all adult patients who received a renal transplant in the Republic of Ireland between 1990 and 2004. Data collected included era of transplantation (1990–1994, 1995–1999, 2000–2004), donor and recipient age and gender, number of human leucocyte antigen mismatches, cold ischemia time (CIT), number of prior renal transplants, immunosuppressive regimen used and acute rejection episodes. Estimated GFR was calculated at 5 years after transplantation from patient data using the Modified Diet in Renal Disease (MDRD) equation. Consecutive sampling was used to divide the study population into two equal unbiased groups of 489 patients. The first group (derivation cohort) was used to construct a predictive model for eGFR five years’ post-transplantation, the second (validation cohort) to test this model. Results: Nine hundred and seventy eight patients were analyzed. The median age at transplantation was 43 years (range 18–78) and 620 (63.4%) were male. One hundred and seventy five patients (17.9%) had received a prior renal transplant. Improved eGFR at five years’ post-transplantation was associated with tacrolimus-based combination immunosuppression, younger donor age, male recipient, absence of cytomegalovirus disease and absence of acute rejection episodes as independently significant factors (p < 0.05). The predictive model developed using these factors showed good correlation between predicted and actual median eGFR at five years. The model explained 20% of eGFR variability. The validation model findings were consistent with the derivation model (21% variability of eGFR explained by model using same covariates on new data). Conclusion: The predictive model we have developed shows good correlation between predicted and actual median eGFR at five years’ post-transplant. Applications of this model include comparison of current and future therapy options such as new immunosuppressive regimens.
Introduction
Patients with end-stage kidney disease have improved quality of life and increased overall survival after renal transplantation compared to those who remain on long-term dialysis treatment.Citation1 Advances in all aspects of transplantation including antimicrobial prophylaxis, surgical technique and immunosuppression regimes have significantly improved short-term renal allograft outcomes.Citation2,Citation3 However, improvements in long-term outcomes have been more modest. A predictive model for long-term allograft function would be very useful in that it would provide important prognostic information.
Several clinical factors such as recipient and donor age, the donor and recipient cytomegalovirus (CMV) disease, presence of acute rejection episode and estimated glomerular filtration rate (eGFR) at one year have been reported to be predictors of the long-term survival of renal allograft.Citation4,Citation5 The purpose of this study was to examine in more detail the influence of pretransplant clinical factors on long-term (5-year) function of deceased donor kidney transplants and to develop a model to predict eGFR based on significant pretransplant factors and to validate this model.
Patients and methods
Patients
This study was a retrospective analysis of all adult deceased donor renal transplants performed in Beaumont Hospital between January 1990 and December 2004. Beaumont Hospital is the national renal transplant centre for the Republic of Ireland. The centre has performed an average of 135 renal transplants per annum over the last 20 years. Patients who underwent primary or repeat deceased donor renal transplantation during this period were included in the study. Exclusions from the study were recipients less than 18 years and simultaneous kidney–pancreas transplants. The data was collected from the Irish Renal Transplant Registry.
The maintenance immunosuppression used in the study period was Cyclosporine (4 mg/kg twice daily) with maintenance trough level of 120–180 ng/mL 6 months post-transplant, Azathioprine (2 mg/kg once daily), Tacrolimus (0.1–0.2 mg/kg/day in two divided doses) with trough level 5–8 ng/mL 3 month post-transplant, Mycophenolate Mofetil (1.5–2.0 g daily) and Prednisolone with maintenance dose of 2.5–5 mg daily. The main induction therapy used was intravenous methylprednisolone 500 mg daily for 3 days, ATG (Anti-Thymocyte Globulin) which was only used for high risk patients (recipients with higher percentage of generated PRA) and Basiliximab which was introduced in 2004.
The study population was divided into three eras according to the time of transplantation (1990–1994, 1995–1999 and 2000–2004). We analyzed the influence of clinical and demographic factors on eGFR at 5 years’ post-transplantation. Of the patients transplanted a total of 978 transplants were functioning after 5 years and the eGFR of each transplanted patient was recorded. eGFR was calculated using the Modified Diet in Renal Disease (MDRD) equation.Citation6 eGFR was chosen as it has been shown to be a more accurate indication for future graft function and survival than serum creatinine levels alone.Citation7,Citation8
MDRD equation was utilized for this analysis as it remains the most widely utilized equation for the estimation of GFR in clinical practice across the world. However, it has certain limitations and its reliability and accuracy decreases in extremes of GFR like in healthy adult and patients on dialysis. Also variation in muscle mass or diet may underestimate GFR using MDRD equation.
Predictors
The demographic and peri-transplant factors studied were era of transplantation, age and sex of recipient and donor, time spent on dialysis prior to transplantation, number of prior transplants, panel reactive antibody status (PRA), cold ischemia time (CIT) and human leucocyte antigen (HLA) mismatching. Post-transplant factors assessed were the immunosuppression regimen, presence or absence of delayed graft function, acute rejection (defined as biopsy proven acute cellular rejection within 3 months post transplant) and CMV disease (defined as active CMV disease for which the individual was receiving intravenous ganciclovir as treatment).
Statistical analysis
Consecutive sampling was used to select two equal groups of 489 patients. A derivation group was used to determine clinical variables that significantly affected eGFR at 5 years’ post-transplant. A validation group was used to repeat this exercise to confirm significant clinical variables. A test predictive model was developed based on the significant variables and tested using the validation group patients. Consecutive sampling was based on date of transplant and recruitment into the two groups was determined by alternately grouping successive transplants. Patients transplanted on the same day were randomly chosen to be in each group. This procedure was considered the most practical and efficient means of sampling compared to a computer generated “pseudo” random sample.
Wilcoxon Rank sum tests and Pearson Chi squared tests were used to compare demographic variables in the derivation and validation cohorts. The Kaplan Meier method determined survival outcomes and a log rank test was used to compare survival outcomes between eras. Log of eGFR at 5 years was modeled using analysis of variance (ANOVA) on various influential variables mentioned above. Variability of the model (model sum of squared) was compared with the variability of the residuals (residual sum of squares). Corrections to the sum of squares totals were made according to the degrees of freedom (DF) to obtain the mean sum of squares. The extent to which the model explained the eGFR variability was determined with the corrected R-squared statistic where correction in this case was for the number of variables used in the model. A parsimonious set of predictors of eGFR at 5 years was derived using stepwise backwards regression. Adjusted R squared determined the variability of eGFR outcome that is attributable to the model covariates. Software used was Stata, version 10 (College Station, TX). A p value < 0.05 was deemed to be significant.
Results
Baseline characteristics
Between January 1990 and December 2004 there were 1975 renal transplants performed. Demographic detail of overall patients and the two patient cohorts were recorded and presented in . The derivation and validation cohorts were similar at baseline.
Table 1. Demographic results of predictive and test model cohorts.
Graft survival
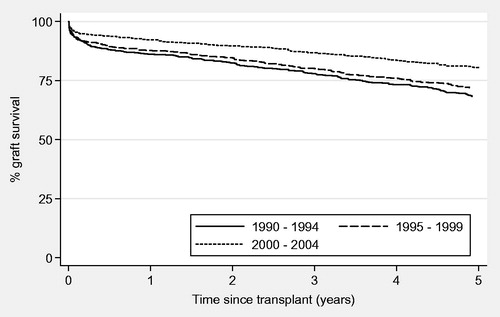
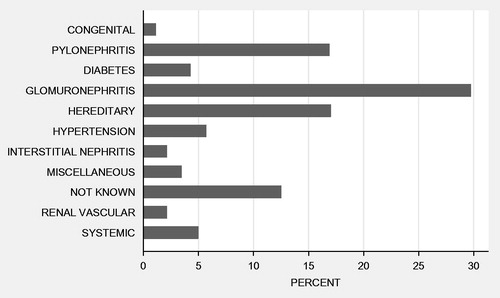
Overall graft survival in the study population at 1 and 5 years post-transplant was 89% and 74%, respectively. For eras 1–3 the rates were 86%, 88% and 92% for 1-year graft survival and 69%, 72% and 81% for 5-years graft survival. There was a significant difference in graft survival between different eras (p < 0.001; ). Causes of original end-stage kidney disease are presented in .
Model derivation
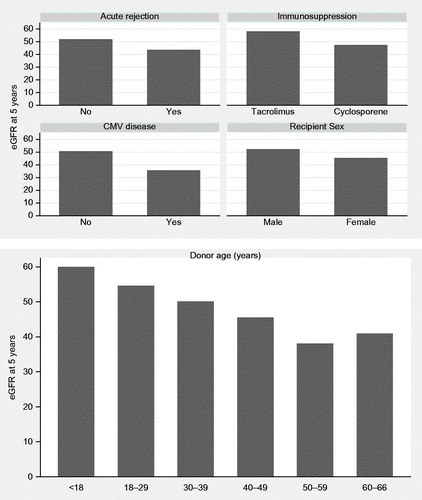
Factors significantly affected eGFR at 5 years were donor age (p < 0.001), recipient sex (p = 0.001), episodes of acute rejection (p = 0.006), CMV disease (p = 0.016) and immunosuppression regimen used. Tacrolimus-based regimen compared to cyclosporine-based immunosuppression regimen was significantly associated with improved eGFR at follow-up (p = 0.015; ). The difference in eGFR at 5 years post-transplant for categories of the significant variables is presented in . A prediction model comprising these significant variables explained 20% of the variability of eGFR at 5 years post-transplant (adjusted R2 = 0.1998 ).
Table 2. ANOVA model for derivation cohort.
Table 3. Reduced ANOVA model for derivation cohort.
Model validation
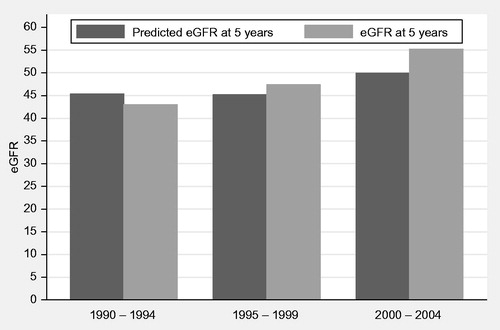
A reduced derivation model was used to predict variability of eGFR at 5 years in the validation cohort. This validation cohort produced similar results to the derivation cohort. All of the variables were significant, apart from CMV disease (p = 0.115; adjusted R2 was 0.2052 or 21% of variability predicted by model). A comparison of predicted and actual eGFRs is presented by era of transplantation in .
Table 4. ANOVA model for validation cohort.
Discussion
Since the first report on renal transplantation in 1955, there has been a continuing effort to improve the short- and long-term survival of renal transplants.Citation9 In this study we focused on clinical factors at the time of transplantation (and early post-transplant period) that could potentially affect eGFR at five years and have an impact on long-term survival of the graft. The factors chosen have been shown to be useful in the prediction of graft function over shorter periods of time.Citation10
The overall graft survival seen in our transplant population compares favorably with international data. This centre is the national transplant centre which performs all renal transplants in Ireland. This gives us large numbers to analyze in a study. Furthermore, patient management is uniform according to single centre management guidelines. These qualities allow for better assessment of factors effecting eGFR at 5 years and also easy extrapolation of our results to other centers.
Advanced donor age, in our study, was found to be associated with low eGFR five years post-renal transplantation. Studies have also identified advanced donor age as a risk factor for delayed graft function (DGF), which is an independent predictor of late graft outcome and graft failure.Citation11 An increase in the donor's age is also found to be associated with a greater incidence of chronic allograft nephropathy (CAN) and worst graft survival.Citation12 Cytomegalovirus (CMV) is the single most important infectious agent in kidney allograft recipients and is a major source of morbidity.Citation13 CMV infection and disease were found to be independent risk factors for clinical acute rejection during the first 100 days post-transplantation.Citation14,Citation15 The impact of early CMV infection and disease on long-term outcome after transplantation remains controversial.Citation16–18 In this study we show that CMV disease has a significant effect on eGFR 5 years post-transplant. However, whether it has an effect on long-term graft survival remains unknown.
Tacrolimus, as an independent factor from acute rejection, was also shown to positively impact long-term graft function. Interestingly when immunosuppressive agents were compared, use of tacrolimus was associated with the higher median eGFR at 5 years post-renal transplantation (56.2 compared to 47.2 for cyclosporine-based regimen). The era effect was not independently significant when immunosuppression was added to the model as in , although median eGFR in the early 1990s, when tacrolimus was not widely used was 45.6 (transplant era 1990–1994). This median value improved to 54.8 once tacrolimus became more widely used (era 2000–2004) which is shown in .
Occurrence of acute rejection in this study was also identified as a factor that affects eGFR at 5 years post-transplant. In previous studies acute rejection has been shown to be one of the strongest negative prognostic factors for long-term graft survival after kidney transplantation.Citation19–23 These studies implied that a reduction in early acute rejection rates would lead to improvements in long-term graft survival.Citation17 It is worth noting that delayed graft function and CIT in this study did not show any statistically significant effect on graft function at 5 years post-transplant. This would suggest the effect of delayed graft function was reduced over the period of the study as it is known to affect function and survival over shorter periods of follow-up.Citation24 It has also been shown that histological damage done due to delayed graft function and CIT does not have a lasting effect on function.Citation25 CITs of the patients included in the study showed relatively minimal variance between groups which could account for its lack of influence.Citation26
Currently there is increasing demand for kidney transplantation. This has led to an increased use of expanded criteria donors, which include all donors older than 60 years and donors older than 50 years with any two of the following criteria: hypertension, cerebrovascular cause of brain death, or terminal serum creatinine (SCr) level >1.5 mg/dL. Our predictive model can be used to guide organ allocation strategies. This model can be utilized to predict eGFR at 5 years. For example, eGFR at 5 years for a male recipient with donor age <18 years, who had no CMV disease, no acute rejection episode and had tacrolimus-based immunosuppression is predicted as 71.9 mL/min. Whereas eGFR at 5 years for a female recipient with donor age >50, who had an acute rejection episode, CMV disease and received cyclosporine-based immunosuppression is predicted to be 23.7 mL/min. The application of similar predictive models using other parameters to assess the benefit of novel therapies has been attempted over shorter time periods.Citation27
Overall models predicting graft function over one year using histological criteria have also been tested and shown to have poor correlation to actual function at one year.Citation28 The authors believe that the inclusion of further factors not specifically linked to transplantation may increase accuracy to a suitable level. GjertsonCitation29 and Ortiz et al.Citation30 demonstrate the distinct influence of factors such as systolic and diastolic blood pressure, hyperlipidemia and glycemic control on both graft function and survival. With the inclusion of these factors a practical predictive model for five year follow-up may be possible but would require further study – Anglicheau et al.Citation31 came to a similar conclusion using histopathological factors in marginal donors.
Our study has several limitations. It has limitations inherent to any single centre study. Also there may be other clinical factors, such as donor cause of death, which we have not included in our predictive model, but are known to influence kidney transplant outcomes. The model does not incorporate other post-transplantation variables that have the potential to impact on long-term graft function such as blood pressure control and post-transplant diabetes.
Conclusion
The advancements in the management of deceased donor renal transplant recipients over the last 20 years have led to marked improvement in transplantation outcomes. This is due in part to reduced episodes of acute rejection and improved graft function. Combination immunosuppressive therapy including tacrolimus as opposed to cyclosporine shows a clear benefit at five years in allograft function. Finally, the development of a predictive model for graft function at five years’ post-transplantation is a potentially fruitful endeavor, but improvements in the accuracy of such a model would be required through the addition of further criteria.
Declaration of interest
The authors of this manuscript have no conflicts of interest to disclose.
References
- Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730
- Woodward RS, Kutinova A, Schnitzler MA, et al. Renal graft survival and calcineurin inhibitor. Transplantation. 2005;80(5):629–633
- Ahsan N, Johnson C, Gonwa T, et al. Randomized trial of tacrolimus plus mycophenolate mofetil or azathioprine versus cyclosporine oral solution (modified) plus mycophenolate mofetil after cadaveric kidney transplantation: Results at 2 years. Transplantation. 2001;72(2):245–250
- Hariharan S, McBride MA, Cherikh WS, et al. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int. 2002;62:311–318
- Resende L, Guerra J, Santana A, et al. First year renal function as a predictor of kidney allograft outcome. Transplant Proc. 2009;41(3):846–848
- Dehnen D, Quellmann T, Herget-Rosenthal S. Current equations estimating glomerular filtration rate in primary care: Comparison and determinants. Scand J Urol Nephrol. 2012;46(6):448–453
- Lenihan CR, O'Kelly P, Mohan P, et al. MDRD-estimated GFR at one year post-renal transplant is a predictor of long-term graft function. Ren Fail. 2008;30(4):345–352
- Magott-Procelewska M, Boratynska M, Janczak D, et al. Estimated glomerular filtration rate evolution between 6 and 24 months predicts long-term kidney transplant survival among patients with inferior graft function. Transplant Proc. 2009;41(8):3028–3032
- Hume DM, Merrill JP, Miller BF, et al. Experiences with renal homotransplantation in the human: Report of nine cases. J Clin Invest. 1955;34(2):327–382
- Moore J, Tan K, Cockwell P, et al. Predicting early renal allograft function using clinical variables. Nephrol Dial Transplant. 2007;22(9):2669–2677
- Moreso F, Seron D, Gil-Vernet S, et al. Donor age and delayed graft function as predictors of renal allograft survival in rejection-free patients. Nephrol Dial Transplant. 1999;14(4):930–935
- Oppenheimer F, Aljama P, Asensio Peinado C, Bustamante Bustamante J, Crespo Albiach JF, Guirado Perich L. The impact of donor age on the results of renal transplantation. Nephrol Dial Transplant. 2004;19(Suppl 3):iii11–iii15
- Rubin RH. Impact of cytomegalovirus infection on organ transplant recipients. Rev Infect Dis. 1990;12(Suppl 7):S754–S766
- Sagedal S, Nordal KP, Hartmann A, et al. The impact of cytomegalovirus infection and disease on rejection episodes in renal allograft recipients. Am J Transplant. 2002;2:850–856
- Solbjorg Sagedal, Andres Hartmann, Knut P.Nordal, et al. Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney Int. 2004; 66:329–337
- Giral M, Nguyen JM, Daguin P, et al. Mycophenolate mofetil does not modify the incidence of cytomegalovirus (CMV) disease after kidney transplantation but prevents CMV-induced chronic graft dysfunction. J Am Soc Nephrol. 2001;12:1758–1763
- Humar A, Gillingham KJ, Payne WD, et al. Association between cytomegalovirus disease and chronic rejection in kidney transplant recipients. Transplantation. 1999;68:1879–1883
- Schnitzler MA, Lowell JA, Hmiel SP, et al. Cytomegalovirus disease after prophylaxis with oral ganciclovir in renal transplantation: The importance of HLA-DR matching. J Am Soc Nephrol. 2003;14:780–785
- Tesi RJ, Elkhammas EA, Henry ML, Davies EA, Salazar A, Ferguson RM. Acute rejection episodes – Best predictor of long-term primary cadaveric renal transplant survival. Transplant Proc. 1993;25:901–902
- Pirsch JD, Ploeg RJ, Gange S, et al. Determinants of graft survival after renal transplantation. Transplantation. 1996;61:1581–1586
- Almond PS, Matas A, Gillingham K, et al. Risk factors for chronic rejection in renal allograft recipients. Transplantation. 1993;55:752–756
- Ferguson RM. Acute rejection episodes – Best predictor of long term primary cadaveric renal transplant survival. Clin Transplant. 1994;8:328–331
- Flechner SM, Modlin CS, Serrano DP, et al. Determinants of chronic renal allograft rejection in cyclosporine-treated recipients. Transplantation. 1996;62:1235–1241
- Moreira P, Sá H, Figueiredo A, et al. Delayed renal graft function: Risk factors and impact on the outcome of transplantation. Transplant Proc. 2011;43(1):100–105
- Nankivell BJ, Fenton-Lee CA, Kuypers DR, et al. Effect of histological damage on long-term kidney transplant outcome. Transplantation. 2001;71(4):515–523
- Van der Vliet JA, Warlé MC, Cheung CL, et al. Influence of prolonged cold ischemia in renal transplantation. Clin Transplant. 2011;25(6):E612–E616
- Schnitzler MA, Lentine KL, Axelrod D, et al. Use of 12-month renal function and baseline clinical factors to predict long-term graft survival: Application to BENEFIT and BENEFIT-EXT trials. Transplantation. 2012;93(2):172–181
- Moscoso-Solorzano GT, Mastroianni-Kirsztajn G, Ozaki KS, et al. Are the current chronic allograft nephropathy grading systems sufficient to predict renal allograft survival? Braz J Med Biol Res. 2008;41(10):896–903
- Gjertson DW. A multi-factor analysis of kidney regraft outcomes. Clin Transplant. 2002:335–349
- Ortiz F, Paavonen T, Törnroth T, et al. Predictors of renal allograft histologic damage progression. J Am Soc Nephrol. 2005;16(3):817–824
- Anglicheau D, Loupy A, Lefaucheur C, et al. A simple clinico-histopathological composite scoring system is highly predictive of graft outcomes in marginal donors. Am J Transplant. 2008;8(11):2325–2334