Abstract
The complement system plays a vital role in preventing life-threatening infections by ensuring optimal functioning of the host immune system. Its dysregulation has been implicated in causing glomerular, hematological, and transplant-related disorders. Eculizumab a novel monoclonal antibody against complement component C5 has emerged in the recent past as the standard of care offering an effective rescue and maintenance therapy against many of these disorders. Its use has been associated with increased risk of infections predominantly with encapsulated organisms. There is no data in the literature on its effects in end-stage kidney disease (ESKD) or dialysis patients. We describe here a very rare case of Aspergillus Niger peritonitis in an ESKD patient on peritoneal dialysis (PD) receiving maintenance eculizumab therapy for atypical hemolytic uremic syndrome. Given that murine models with the same defect as that induced by eculizumab is vulnerable to invasive Aspergillosis, it is suggested that the fungal peritonitis in this patient was the result of the eculizumab therapy.
Introduction
Peritonitis is a major cause of peritoneal dialysis (PD) technique failure, accounting for 15–35% of PD hospital admissions. Fungal peritonitis (FP) is rare and estimated to account for 3–6% of all PD peritonitis.Citation1 It is associated with poor outcome. A recent review noted a hospitalization rate of 98%, catheter removal rate of 88%, while 74% of patients needed permanent transfer to hemodialysis. Candida species accounts for 70–90% of FP in adults and 80–100% in the pediatric population.Citation1
Aspergillus is a filamentous mould omnipresent in the environment. FP due to Aspergillus species is extremely rare and carries a very high mortality rate ranging from 15% to 50%.Citation2 The reported incidence of peritonitis due to Aspergillus niger is 2.2% of all the fungal peritonitis.Citation3 Risk factors for Aspergillus peritonitis include prolonged antibiotic therapy, corticosteroid therapy, recent bacterial peritonitis, recent hospitalization, and lupus.Citation3–5 The conidia of pathogenic Aspergillus species are extremely small (2–5 µm)Citation6 and entry into the peritoneal cavity has been reported to occur through the catheter intraluminally, periluminally, and rarely by the vaginal route.Citation5 The PD catheter is usually colonized, so catheter removal is necessary in addition to antifungal therapy. The high mortality has been attributed to the delayed diagnosis and the subsequent delay in surgical removal of the catheter largely due to a low index of suspicion from non-specific symptoms or from drainage problems as a result of an Aspergillus mass in the catheter lumen.Citation6,Citation7
The complement system comprises a group of proteins that, when activated, brings in a synergy between innate and adaptive immune systems providing a robust defense against microbes. This is ensured through opsonization, local inflammation and chemotaxis, cell lysis, and subsequent clearance of immune complexes all in a finely regulated way.Citation8 Opsonization employs the deposition of large amounts of C3b and its subsequent active products on the microbe while the local inflammation and chemotaxis is largely ensured by anaphylotoxins C3a and C5a, thus ensuring a functional native immunity. Simultaneous activation of the innate immunity occurs through antigen presentation, costimulation of B lymphocytes with subsequent antigen memory retention by the immune system through activated complement products and their mediators of inflammation.Citation6,Citation8
Dysregulation of the complement pathway is associated with various forms of glomerulonephritis and atypical hemolytic uremic syndromes (aHUS). Eculizumab is a monoclonal antibody against human complement component 5 (C5) and blocks cleavage of C5, thus inhibiting formation of C5a, C5b-9, and the membrane attack complex (MAC). It is now considered as first-line therapy against aHUS. Lifelong therapy with eculizumab for aHUS is now approved as a standard of care. Activated terminal complement factors form the MAC, which ensures the bactericidal activity especially against encapsulated organisms through a perforin-like action on the bacterial cell wall leading to cell lysis. Vaccination against the encapsulated organisms Neisseria meningitidis, Streptococcus pneumonia, and Hemophilus influenza type B has been recommended along with continuous antibiotic prophylaxis for the treated patients owing to increased susceptibility to infections.Citation9 We present a case of A. Niger peritonitis in a PD patient on eculizumab treatment.
Case report
The patient is a 19-year-old female, the youngest of four siblings. She was first evaluated for a flu-like syndrome and hematuria at 6 months of age and was diagnosed to have aHUS. Subsequent evaluation revealed a membrane cofactor protein (MCP) gene mutation to be the cause of her aHUS. Her course was complicated by one episode of acute kidney injury with seizures at 4 years of age and responded to plasma exchange. Subsequently she was in remission with periodic plasma infusions and had a stable course until 14 years of age when she had a relapse. She was treated then with plasma exchange and hemodialysis. Unfortunately, she progressed to end-stage kidney disease (ESKD) and continued on hemodialysis by a tunneled catheter for about a year. She switched to PD at 15 years of age and was on continuous cycling PD (CCPD). She continued to require periodic plasma infusions to prevent systemic microangiopathy as a result of aHUS. PD was complicated in the first year by an episode of bacterial peritonitis that responded well to therapy. From her 16th year onwards, she was started on eculizumab after stopping the plasma infusions in view of increasing allergic response to plasma products. She remained in remission on a regimen of 900 mg of eculizumab IV every 2 weeks.
At 19 years of age, she was evaluated for abdominal pain and fever. Her PD fluid analysis revealed a white cell count of 600 with 48% neutrophils, 35% monocytes, 8% eosinophils, and 9% mesothelial cells.
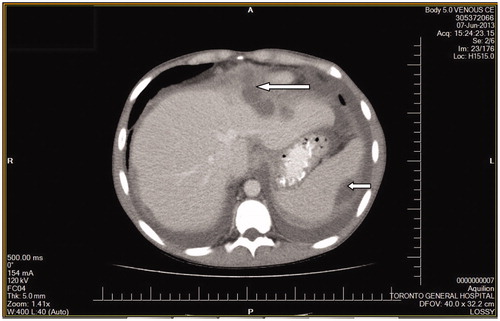
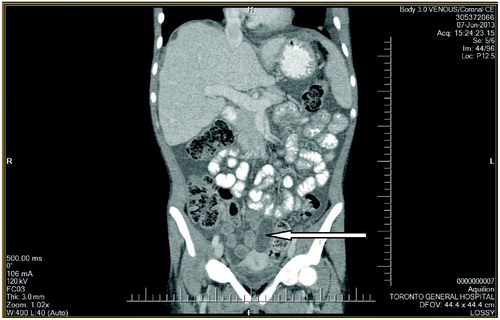
She was allergic to cephalosporins and received initially intra-peritoneal (IP) vancomycin and gentamicin with oral nystatin prophylaxis as per standard guidelines. However, she continued to experience diffuse abdominal pain and was febrile with leucocytosis and persistent cloudy PD fluid. PD catheter was surgically removed in view of the refractory PD peritonitis and she was transferred to hemodialysis. Two of the PD fluid cultures grew A. Niger of which one of the cultures also grew Escherichia coli and Enterococcus faecium. She was started on IV ampicillin and meropenem after infectious diseases (ID) consult and review of culture sensitivity results along with IV amphotericin B with a switch to voriconazole subsequently. Surgical consultation and contrast CT (CCT) scan of the abdomen found no evidence of bowel perforation or any other localized acute pathology. Subsequent CCT of the chest did not reveal any primary focus for Aspergillus. A follow-up CCT abdomen revealed new intra-abdominal collections and these were drained with symptomatic relief ( and ). Eculizumab was discontinued and she was switched to plasma exchange every 3 weeks to prevent relapse of aHUS. She recovered well and is now training for home hemodialysis. This is the first case in the literature of polymicrobial A. Niger peritonitis in a patient on eculizumab therapy.
Discussion
Aspergillus fungal peritonitis in PD is very rare and associated with serious complications. It has been usually described in the background of excessive immune suppression and with conditions contributing to altered host microflora. Aspergillus species activate all the three pathways of the complement system in a sequential manner with the resting conidia activating the alternate pathway, while swollen conidia and the mature hyphae activate the classic pathway. Melanin has been identified as the surface molecule responsible for the activation of the alternative pathway in A. Niger infection.Citation6,Citation8
Fungi, owing to their thick cell wall, are resistant to the membrane attack complex (MAC) of the complement cascade. Opsonization with C3-derived fragments plays a major role for efficient phagocytosis, induction of oxidative burst, and destruction of fungi by monocytes.
Eculizumab is a novel monoclonal antibody targeted against the C5 component of the complement pathway. It leads to a functional C5-deficient state through effective blockade of C5 cleavage to subsequent C5a and C5b-9 which mediate the pro-inflammatory, pro-thrombotic, and lytic functions of complement.Citation9 Interestingly, invasive Aspergillosis has been reported in murine models with the same C5 and C5a defects.Citation6,Citation8
Therefore, we suggest that the A. niger PD peritonitis in our patient is likely secondary to the altered host complement system as a result of chronic Eculizumab therapy. While the manufacturer has emphasized the importance of vaccination against Neisseria species, it is possible that the impairment of the complement cascade induced by this drug leads to susceptibility to infection by other organisms.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Matuszkiewicz-Rowinska J. Update on fungal peritonitis and its treatment. Perit Dial Int. 2009;29(Suppl. 2):S161–S165
- Bonfante L, Nalesso F, Cara M, et al. Aspergillus fumigatus peritonitis in ambulatory peritoneal dialysis: a case report and notes on the therapeutic approach. Nephrology. 2005;10:270–273
- Schattner A, Kagan A, Zimhony O. Aspergillus peritonitis in a lupus patient on chronic peritoneal dialysis. Rheumatol Int. 2006;26:762–764
- Raaijmakers R, Schroder C, Monnens L, Cornelissen E, Warris A. Fungal peritonitis in children on peritoneal dialysis. Pediatr Nephrol. 2007;22:288–293
- Bibashi E, Papagianni A, Kelesidis A, Antoniadou R, Papadimitriou M. Peritonitis due to Aspergillus niger in a patient on continuous ambulatory peritoneal dialysis shortly after kidney graft rejection. Nephrol Dial Transplant. 1993;8:185–187
- Speth C, Rambach G, Wurzner R, Lass-Florl C. Complement and fungal pathogens: an update. Mycoses. 2008;51:477–496
- Jeloka TK, Shrividya S, Wagholikar G. Catheter outflow obstruction due to an Aspergilloma. Perit Dial Int. 2011;31(2):211–212
- Kozel TR. Activation of the complement system by the pathogenic fungi. Clin Microbiol Rev. 1996;9(1):34–46
- Zuber J, Fakhouri F, Roumenina TL, Loirat C, Fremeaux-Bacchi V. Use of Eculizumab for atypical hemolytic uremic syndrome and C3 glomerulopathies. Nat Rev Nephrol. 2012;8:643–657