Abstract
There is very little work on the expression of TRPM6/7 in ischemia reperfusion models. In previous studies, after ischemia, reperfusion had been kept limited to 24 h, yet in our study, expressions of these channels were elucidated after its modification to 48 h to establish what kind of changes renal tissues undergo. For the current study, 20 Wistar albino rats were divided into two groups equally. Group I: control group, Group II = I/R group (60 min ischemia + 48 h reperfusion). For the mRNA analysis, right kidneys of I/R group was used as a reference in order to eliminate genetic differences. The left renal artery (I/R generated part) of I/R area was removed from all rats in the second group. Likewise, normal tissues of right renal artery were removed from all rats. Histopathologic scoring of the tissue samples were achieved semi-quantitatively according to normal tissue composition. Consequently, both TRPM6 and TRPM7 expression levels were decreased in all groups according to control groups, yet results were not counted as significant (p > 0.05). Additionally, correlation analysis confirmed these results. Also, I/R performed kidneys had more tissue damage compared to control group. To conclude, our study results suggest that TRPM6/7 expressions may be increased and after 48 h of reperfusion expression levels of these two stored to normal levels. At the same time, damages have occurred in renal tissues after ischemia. These damages were considered to be resulted from the oxidative effects as previously reported.
Introduction
Ischemia and reperfusion (I/R) is a common clinical event in many areas of medicine. I/R is pathophysiological process in which not all molecular and clinic aspects is elucidated enough and involved in almost all medical disciplines such as hypovolemic shock, burns, sepsis, pancreatitis, partial nephrectomy surgery, coronary artery bypass grafting, a number of urological initiatives, after aortic or peripheral arterial clamp administration in cardiovascular surgery, iatrogenically as a result of tourniquet application in vascular, orthopedic and reconstructive surgery, cancer and infection.Citation1 Acute or chronic circulatory disorders, disease states in which organ perfusions affected, ischemic problems after flap surgery, almost every step of solid organ transplantation are the most affected pathologies by this process.Citation2
Failure of the blood supply to organs as a result of insufficient or stopped blood flow to an organ because of various reasons (hypovolemic shock, burns, sepsis, pancreatitis and other hypoxic diseases), so sufficient level of oxygen and nutrients cannot be reached to the tissues and inability of removal of the waste products by circulation in this process, is defined as ischemia. As a result of ischemia tissue remains in hypoxia and hypoxic tissue damage occurs. Ischemia causes cell dysfunction or disruption of cell integrity and ultimately causes cell death by decreasing the energy level in the cell and leading to the accumulation of toxic metabolites.Citation3
Ischemia can lead to cell and/or tissue damage reversibly or irreversibly depending on the insufficiency of blood supply to an organ or tissue.Citation4 Reperfusion is the resumption of blood supply to the tissue or organ to meet the emerging energy requirement and for removal of toxic metabolites as a result of ischemia.Citation5 Reperfusion of ischemic tissue paradoxically leads to more damage than ischemic damage.Citation6
Several associated factors have been suggested in the pathophysiology of ischemia reperfusion injury. These relationships are complex series of cellular and humoral events.Citation7 In particular: (1) free oxygen radicals, (2) polymorphonuclear leukocytes (PMNL), (3) complement system, (4) the endothelial cells are the four factors involved in the main causes of damage. There are two main mechanisms for the formation of the reperfusion injury. First one them is the release of the superoxide radicals (SOR), and the other one is the breakdown of fatty acids in membranes by a hydrolytic enzyme phospholipase A2 which is activated by Ca + 2 in ischemic period.Citation8 As a result of reperfusion blood and oxygen is supplied to the tissue. The removal of hypoxanthine accumulated during ischemia in tissue is attempted. In the presence of oxygen, xanthine oxidase is activated to form SOR. Increase in the level of SOR is leading to cell dysfunction by lipid peroxidation and protein degradation and ultimately resulting in tissue necrosis. Free oxygen radicals, both directly damaging the tissue and leads to the accumulation of PMNL in damaged tissue. Considering the active PMNL to tissue is releasing enzymes such as myeloperoxidase (MPO), elastase, proteases and collagenase. These enzymes are increasing the damage in the tissue and cause the formation of more SOR.Citation9
Calcium is a vital essential element for humans and all mammalian cells. However, accumulation of calcium overload in cells causes excessive activation of the signaling processes that leads to cell death.Citation10 However, the mechanism of cytotoxic activity of calcium is still a controversy.Citation11 Although there are several different ways involved in the entry of calcium in cells,Citation11 TRPM channels is one of the most important ones. These channels are divided into eight sub-channels (TRPM1-8). It has been reported that these channels lead to cell death via anoxia and the formation of reactive oxygen species. Overexpression of these channels leads to cell death.Citation12 Especially, in conditions where TRPM7 is overexpressed in kidneys, cell deaths and tissue damages have been reported.Citation13 Like calcium, magnesium is also an important element. TRPM6 plays an important role in the uptake of magnesium. In conditions where TRPM6 levels decreased, important renal and intestinal dysfunctions have been reported.Citation14 Nonetheless, there is not much work on TRPM6 expressions in ischemia reperfusion models. In previous studies, reperfusion is limited to 24 h after ischemia. In the present study, expression levels of these channel proteins were revealed after modifying the reperfusion duration to 48 h to investigate pathophysiological changes in renal tissues.
Materials and methods
This study was approved by an experimental animal ethics committee (02/08/2013/170) and held at the experimental animal laboratory at Mustafa Kemal University, Faculty of Medicine. Histopathologic examinations were held at the Department of Pathology, Mustafa Kemal University, Faculty of Medicine. RNA isolation and expression studies were held in Molecular Genetics Diagnostic Laboratory of Sahinbey Training and Research Hospital, Gaziantep University, Faculty of Medicine. In this study, total number of 20 adult male Wistar albino rats weighing 200–250 g was used. Prior to the experiment all animals were held in circadian rhythm for 12 h day and 12 h night in 24–26 °C ambient temperature and 50–60% humidity. The rats were fed by a standard commercial pellet feed and top water.
Group I: Control group – No treatment provided to the subjects in this group. Evaluated as a control group. Kidney tissue samples were obtained from these subjects after anesthesia by applying laparotomy.
Group II: I/R group – No treatment provided to the subjects in this group. After laparotomy, by placing atraumatic vascular clamp to bilateral renal artery 60 min ischemia was performed.Citation15,Citation16 After 48 h reperfusion, all of the subjects of the kidney tissue samples were collected by laparotomy. For genetic analysis, left and right kidneys are removed in the ischemia group. Right kidneys were used as an internal control group.
10 mg/kg xylazine (Rompun, Bayer, Turkey) and 40 mg/kg ketamine (Ketalar, Eczacibasi, Turkey) anesthesia (i.p.) were applied intraperitoneally (i.p.) to all subjects. Abdominal area of all subjects was shaved and sterilized with 10% polyvinylpyrrolidone–iodine complex (Batticon, Adeka). Approximately 3 cm incision was made on the midline of the abdominal area. Abdominal cavity once made visible and abdominal organs were taken towards the edges. Thus, renovascular bed was made visible. Without applying any process to the control group, after peduncle of the left kidney made visible one atraumatic clamp was applied for 60 min and removed in the I/R group. After clamp administration discoloration was observed in kidneys. Sponge moistened with the warm physiological saline was placed on exposed abdominal regions during 60 min reperfusion in all groups. In subjects, in which 48 h reperfusion was applied, laparotomy incision was closed by primer 3/0 silk suture after ischemia. Subjects were anesthetized again after 48 h and laparotomy incisions were opened. Also, blood samples collected from the hearts of the subjects for biochemical parameters. After removal of the capsule collected kidney tissue divided into two by scalpel through longitudinal incision. One of the parts was placed into 10% formalin solution for histopathologic examinations. The remaining part was kept in −80 °C until RNA isolation.
Preparation of histopathologic kidney samples
For histopathologic examinations, after fixation of kidney samples that are kept in 10% formalin. routine histologic paraffin block preparation method were followed. 5-μm thick sections were obtained from paraffin blocks by using a microtome (Leica Rotary; Leica Microsystems GmbH, Wetzlar, Germany). Collected sections were stained with hematoxylin–eosin (H&E) and examined by visualizing under 100 × magnification in light microscope and photos were taken. Histopathology of the tissue samples were scored semi-quantitatively according to normal tissue composition. In the pathological examinations of the sample parameters of brush border loss, extravasation, tubular cast structures, nucleus loss in the tubule epithelial cells, tubular dilatation and interstitial accumulation of lymphocytes, tubular necrosis were scored in between 0 and 5; thus, normal kidneys and ischemia/reperfusion applied kidneys were compared. This scoring (0: normal tissue, 1: blown tubular epithelium cell areas, vacuolar degradation, necrosis less than 25% of cases, 2: 25–50% of similar cases, 3: 50–75% of cases similar, 4: more than 75% of similar cases, 5: complete cortical necrosis) was carried out according to similar scoring of the ischemia/reperfusion studies in kidney.Citation17
Isolation of total RNA from tissue samples and cDNA preparations
A total of 25 mg of tissue samples were homogenized by Qiazol Lysis Reagent (Qiagen Sample and Assay Technologies, Hilden, Germany) at Qiagen Tissue Lyser (Qiagen Sample and Assay Technologies, Hilden, Germany) homogenizator. Total RNA isolation of homogenized samples were achieved by using Qiagen RNeasy Mini Kit (Qiagen Sample and Assay Technologies, Hilden, Germany) and maintained at −80 °C till use. NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA) was used to measure RNA integrity. cDNA synthesis was carried out using opsogen RT Kit (Qiagen Sample and Assay Technologies, Hilden, Germany). Reactions were prepared according to manufacturer’s protocols as follows; a pre-denaturation of sample RNA was attained by incubating 5 min at 65 °C and subsequently removed to ice block. Then, the reaction mix (2.5 µl reverse transcriptase 10 × buffer, 2.5 µl dNTP, 5.25 µl random nonamer, 0.5 µl RNAse inhibitor versus 0.5 µl reverse transcriptase) was prepared and distrusted to each reaction tube. Reaction mixtures were completed to 25 µl dH2O and incubated at the following thermal parameters; 60 min at 42 °C, 5 min at 85 °C. Prepared cDNAs were stored at −20 °C until reverse-transcriptase polymerase chain reaction (RT-PCR) quantifications.
Reverse-transcriptase PCR quantifications
Prior to RT-PCR quantifications optimum PCR parameters were determined by using Corbett Research (Model: RG-600, Australia) gradient PCR. RT-PCR quantifications were carried out using ABI 9700 Thermal Cycler (ABI Inc., Foster City, CA). RT-PCR products were run through 2% agarose gel electrophoresis and banding patterns were visualized with an ethidium bromide staining. Integrated density measurements of bands of the gels were assessed using ImageJ v1.46 r program (Wayne Rasband National Institutes of Health, Bethesda, MD).
Statistical analysis
Statistical analysis of resulting data was achieved by GraphPad Prism (v6.02, GraphPad Software, Inc., La Jolla, CA) and SPSS (v16.0, IBM Corporation, Armonk, NY) package programs using an one-way ANOVA test. All expression quantities were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The degrees of significance of differences between groups were assessed by the Fisher’s least significance difference test. All statistical tests were two-tailed and p < 0.05 was considered as significant.
Results
Evaluation of TRPM gene expressions
To reveal the impacts of TRPM6 and TRPM7 genes on ischemia-reperfusion models expression levels were measured by semi-quantitative PCR. Both TRPM6 and TRPM7 expression levels were decreased in all groups relative to the control group yet these changes were not statistically significant (p > 0.05). Additionally, these results are confirmed as the results of correlation analysis ().
Table 1. Gene expression levels of TRPM6 and TRPM 7 in control and application groups.
Histopathologic evaluations
In order to investigate pathological changes in renal tissues after ischemia-reperfusion applications, histopathologic examination of tissue samples were stained with hematoxylin–eosin and scored semi-quantitatively according to overall tissue composition. Resulting observations are presented in . In I/R performed kidneys more tissue damage were observed compared to the control group as shown in .
Figure 1. Microscopic images of (A) control kidney, (B) non-ischemic right kidney, (C) ischemic left kidney (hematoxylin–eosin (H&E) 100×).
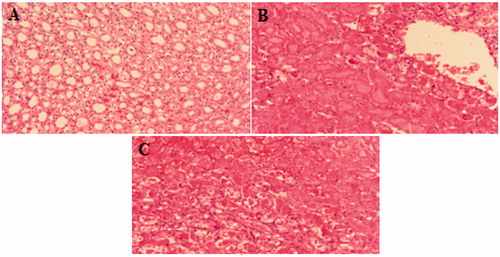
Table 2. Histopathologic scores of ischemic and non-ischemic groups.
Discussion
Besides being important for many living species calcium is also an essential element for humans. Calcium channels have significant roles in the action of this element. Calcium channels (TRPM) act on a variety of crucial functions including ischemia.Citation16 Increasing evidence suggests that, TRPM channels play important roles in ischemia reperfusion.Citation18 These channels have been implicated in many tissue defects as reported in previous studies. In this study, TRPM7 and TRPM6 and gene expression levels were determined from tissue samples of subjects after 60 min ischemia and 48 h reperfusion. Also, isolated organs were examined histopathologically. As a result of the study, both TRPM7 and TRPM6 were found to be down regulated in the experiment group compared to the controls. However, this reduction was not statistically significant. Additionally, expression of these channels was found to be up-regulated in left kidneys of the experiment group compared to right kidneys. Nevertheless, this change was not considered as significant according to statistical analysis. Previous studies reported increased TRPM7 levels in renal system after 24 h ischemia reperfusion application,Citation13 yet our study results revealed no change in the expression of this channel protein after 48 h ischemia reperfusion application. Such differences can be attributed to difference of the methods used, as may also occur due to different tissues. However, TRPM7 known to increase at 24 h as reported renal ischemia/reperfusion studies. In our study, histopathologic tissue damage after 48 h and unchanged TRPM7 levels may be explained by restoring the TRPM7 levels to normal physiological levels in 48 h reperfusion. TRPM7 and TRPM6 are known to form a unique pair of channels. While TRPM6 is a calcium channel, it is also involved in magnesium transport. A missense mutation in the coding region of the TRPM6 gene is characterized by the reduced expression of this protein and subsequently decreased the magnesium uptake.Citation14 The role of magnesium in renal system is not fully understood but hyper-or hypo-magnesium levels are known to create significant kidney problems. According to our literature searches, the role of TRPM6 in renal ischemia-reperfusion has not been elucidated yet.
The current study can be considered as the first report on TRPM6/7 expressions after 48 h reperfusion in renal system. In previous ischemia-reperfusion studies,Citation16,Citation19 tubular damage, renal tubular dysfunction and tubular cell necrosis was reported in variety of tissues. In our study, extravasation, nucleus loss in epithelial cells, tubular dilatation, tubular necrosis and interstitial lymphocyte accumulation were observed ().
In the renal tissue, released MDA amount due to oxidative stress has been reported to increase after ischemia-reperfusion and this increase has been reported to result from H2O2.Citation20 Nonetheless, free oxygen radicals are known to be involved in ischemic acute renal failure.Citation21
Taken together, our study results revealed that expressions of TRPM6 and TRPM7 channel proteins were found to be close to that of initial levels after 48 h of reperfusion. This result suggests that levels of these protein channels have increased and after 48 h of reperfusion decreased to normal levels in cells. Lastly, tissue damages may also be resulted from oxidative effects as reported previously.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Plestina S, Gamulin S. Kidney ischemia-reperfusion injury and polyribosome structure. Nephron. 2001;89(2):201–207
- Siemionow M, Arslan E. Ischemia/reperfusion injury: a review in relation to free tissue transfers. Microsurgery. 2004;24(6): 468–475
- Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am. 1992;72(1):65–83
- Kandilci HB, Gümüşel B. Akciğerlerde iskemi-reperfüzyon hasarı ve iskemik önkoşullama. Hacettepe Üniversitesi. Eczacılık Fakültesi Dergisi. 2005;25:35–49
- Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, Piña E, Geller DA. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res. 2008;147(1):153–159
- Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol. 1986;250(6 Pt 1):G749–G753
- Şener G, Yeğen BÇ. Iskemi reperfüzyon hasarı. Klinik Gelişim. 2009;22(3):5–13
- Udassin R, Vromen A, Haskel Y. The time sequence of injury and recovery following transient reversible intestinal ischemia. J Surg Res. 1994;56(3):221–225
- Otamiri T. Oxygen radicals, lipid peroxidation, and neutrophil infiltration after small-intestinal ischemia and reperfusion. Surgery. 1989;105(5):593–597
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391–397
- Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47(2):122–129
- Aarts M, Iihara K, Wei WL, et al. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115(7):863–877
- Meng Z, Wang X, Yang Z, Xiang F. Expression of transient receptor potential melastatin 7 up-regulated in the early stage of renal ischemia-reperfusion. Transplant Proc. 2012;44(5):1206–1210
- Lainez S, Schlingmann KP, van der Wijst J, et al. New TRPM6 missense mutations linked to hypomagnesemia with secondary hypocalcemia. Eur J Hum Genet. 2014;22(4):497–504
- Bulut A, Demir T, Cengiz B, et al. Molecular analysis of Smad-1, Bmp-2, Bcl-Xl and Caspase-3 genes in renal ischemia-reperfusion model in rats. Acta Physiol. 2011;203(Suppl. 686):PC024
- Feng L, Ke N, Cheng F, et al. The protective mechanism of ligustrazine against renal ischemia/reperfusion injury. J Surg Res. 2011;166(2):298–305
- Walker LM, Walker PD, Imam SZ, Ali SF, Mayeux PR. Evidence for peroxynitrite formation in renal ischemia-reperfusion injury: studies with the inducible nitric oxide synthase inhibitor L-N-6-(1-iminoethyl)lysine. J Pharmacol Exp Ther. 2000;295(1):417–422
- Zhang YX, Zhou L, Zhang X, Bai J, Shi M, Zhao G. Ginsenoside-Rd attenuates TRPM7 and ASIC1a but promotes ASIC2a expression in rats after focal cerebral ischemia. Neurol Sci. 2012;33(5):1125–1131
- Rhoden EL, Rhoden CR, Lucas ML, Pereira-Lima L, Zettler C, Belló-Klein A. The role of nitric oxide pathway in the renal ischemia-reperfusion injury in rats. Transplant Immunol. 2002;10(4):277–284
- Chiang WC, Chien CT, Lin WW, et al. Early activation of bradykinin B2 receptor aggravates reactive oxygen species generation and renal damage in ischemia/reperfusion injury. Free Radic Biol Med. 2006;41(8):1304–1314
- Paller MS, Hoidal JR, Ferris TF. Oxygen free-radicals (Fr) in ischemic acute-renal-failure (Arf) in the rat. Kidney Int. 1984;25(1):236–236
