Abstract
Background: Diabetic kidney disease (DKD) is a leading cause of end-stage renal disease (ESRD). Renin–angiotensin–aldosterone system (RAAS) plays a critical role in the development of DKD with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) being the mainstay of treatment. Systemic RAAS activity has been implicated in the pathogenesis of DKD, but lately interest has shifted to intrarenal RAAS effect. With the discovery of the (pro)renin receptor and ACE independent pathways of angiotensin II production, our understanding of role of renin in end organ damage has improved significantly. Summary: We summarize our current understanding of ACE dependent and independent pathways in the development of DKD and the preclinical models demonstrating renal effects of direct renin inhibitors (DRIs). We then review clinical studies and trials performed so far evaluating the efficacy of aliskiren on renal outcomes and safety in DKD. Key message: At present, there is little evidence for renal benefit of aliskiren in DKD beyond that offered by ACEIs or ARBs. Combining aliskiren with ACEI or ARB in DKD did not significantly improve renal outcomes in comparison with ACEI or ARB monotherapy in clinical trials. Slightly more adverse events including hyperkalemia, acute kidney injury and hypotension were observed in the combination therapy as compared to the monotherapy. Thus, current evidence suggests that aliskiren, because of its antihypertensive and antiproteinuric effects, maybe used as monotherapy in DKD and considered an equivalent alternative to ACEIs or ARBs. Careful monitoring for renal adverse effects would allow safe clinical use of DRI.
Introduction
Diabetic kidney disease (DKD) is one of the leading causes of end-stage renal disease (ESRD).Citation1 The critical role of renin angiotensin aldosterone system (RAAS) in the pathogenesis of DKD has been extensively investigated and is very well appreciated. Consequently angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) have been the key component in the management of DKD. More recently, our understanding of local RAAS has improved and hence the role of renin and pro-renin in DKD have received significant attention. Direct renin inhibitors (DRIs) blocks the RAAS at the level of renin and thus are expected to lead to a more complete and sustained blockage of RAAS compared to ACEIs or ARBs and its resultant effects of hypertension and glomerular injury. However, data from clinical trials performed in the last 5 years have not supported a superior effect/role of DRIs compared to ACEIs or ARBs in modulating renal outcomes. We thus performed an up-to-date review of the RAAS to understand the controversies and then review the clinical trials of DRI in management of DKD to provide the reader with the current evidence for the use of DRI and its place in the therapeutic armamentarium for DKD.
Renin
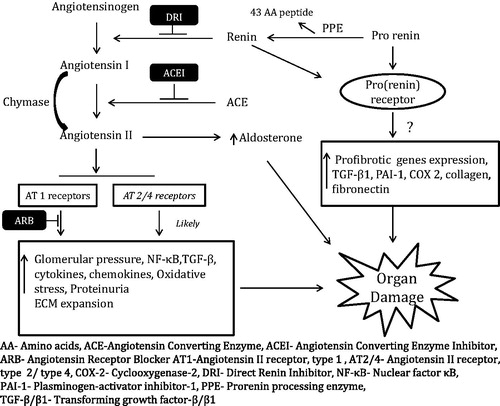
Renin is an aspartase protease formed by two lobules with a cleft in between containing the active site. It is synthesized as a pre–pro-hormone which is converted to pro-renin by cleavage of a 20 amino acid peptide. Pro-renin has a 43-amino-acid pro-segment peptide which covers the cleft thus obstructing the interaction of angiotensinogen to the active site of renin.Citation2 By proteolytic removal of a 43-amino-acid pro-segment peptide from the N-terminus pro-renin is irreversibly converted to active form of renin (). Renin is stored primarily in the juxtaglomerular cell in the afferent renal arterioles and its release is regulated by changes in renal perfusion, plasma volume, sympathetic drive and direct action of angiotensin II on juxtaglomerular cells (JG) cells.Citation2,Citation3
Pro-renin processing enzyme (PPE) is involved in this proteolytic conversion of pro-renin to renin. The exact PPE has not yet been identified but among the potential candidate enzymes cathepsin B was considered the most likely PPE for multiple reasons. This includes the colocalization of cathepsin B with pro-renin in JG cells of animal models and ability of cathepsin B to cleave human pro-renin in vitro at site identified for renin.Citation4,Citation5 But the study done on cathepsin B knockout mice to verify the role of this potential PPE in vivo failed to show its role in proteolytic conversion of pro-renin. These knockout mice had normal levels of circulating renin and also were able to increase renin level equivalent to their wild counter parts on RAAS inhibition by captopril.Citation6 In the light of these results, cathepsin B is not the PPE in mice and given that mice PPE is active on human pro-renin too, cathepsin B is unlikely to be the PPE in humans. So, the search for PPE continues and once identified this can be a potential target for therapeutic interventions at the rate limiting step of RAAS pathway.
RAAS and tissue-specific angiotensin production
Renin when released from JG cells in response to any of the above mentioned stimuli activates the downstream activation of angiotensin and aldosterone as shown in . The conversion of angiotensinogen to angiotensin I is the rate limiting step of RAAS activation and thus makes renin the key enzyme in the whole process.Citation2,Citation3
Local RAAS exists in many organs and tissues and has major significance. Kidneys have all the components of the RAAS and angiotensinogen produced mainly from the proximal tubule cellsCitation7 can be converted to angiotensin I by renin which is filtered and/or secreted from JG cells. Angiotensin I convert to angiotensin II readily as ACE is abundant in the proximal tubule cells.Citation8
The role of local RAAS in extra renal sites is evident from the study showing that ACE-knockout mouse type-2 model inhibited oxidative stress which was based on decrease in lipid peroxide content in their sera and peritoneal macrophages. This mouse model express a truncated form of somatic ACE but completely lacks tissue ACE. APOE-deficient mice develop severe hypercholesterolemia and are commonly used as animal models for atherosclerosis. The crossbreeds of ACE knockout mice type 2 and APOE homozygous-deficient mice exhibited decreased atherogenesis compared with APOE homozygous-deficient mice. These results support that tissue-bound ACE plays an important role in RAAS mediated pathology.Citation9
Apart from the above-mentioned ACE-dependent activation of RAAS pathway, ACE-independent angiotensin II production in various tissues has gained appreciation based on emerging evidence and is discussed later in this review.
Renin-dependent and -independent effects of pro-renin receptor system
With the discovery of the (pro)renin receptor (PRR); a specific receptor for renin and pro-renin, our understanding of role and function of renin in end-organ damage has improved. (Pro)renin receptor is localized primarily on glomerular mesangial cells, distal renal tubule, podocyte and vascular smooth muscle cells.Citation10,Citation11 This receptor binds both renin and pro-renin, which has long been considered only as an inactive precursor of renin. Renin bound to the receptor has higher catalytic activity compared to renin in solution. When pro-renin binds to the PRR, it can also undergo non-proteolytic activation via conformational change and this bound form of pro-renin is as active as renin.Citation10,Citation12 The binding of renin or pro-renin to the receptor triggers intracellular signaling via multiple pathways that upregulates the expression of profibrotic genes.Citation13,Citation14 PRR system is considered to have two functions, an angiotensin-independent function related to PRR-induced intracellular signaling and its downstream effects and an angiotensin-dependent function related to the increased catalytic activity of receptor-bound (pro)renin ().
PRR was initially identified only as a component of RAAS and considered exclusively as an integral membrane protein acting as the receptor of renin and pro-renin. However, recent studies have shown that PRR has wide tissue distribution with angiotensin II independent functions and exist in three different molecular isoforms. Based on animal data it plays a vital role in Vacuolar H + -ATPase function and is co-factor in the Wnt receptor signaling.Citation15 V-ATPases transport protons across plasma membranes in intercalated cells, osteoclasts, macrophages and tumor cells. Wnt signaling is necessary for a normal patterned embryo while in adults, it is involved in cell proliferation, migration and polarity; and abnormality in this signaling promotes degenerative diseases.Citation15 Angiotensin II independent role of PRR is supported by evidence that PRR co-localizes with V-ATPase in synaptic vesicles and in intercalated cells of distal tubule and collecting duct suggesting a link.Citation15,Citation16 Also, PRR knockout mice shows massive cardiac defects attributed to impaired V-ATPase assembly and defective autophagy with possible role of altered Wnt signaling.Citation17 With continuing improvement in the understanding of the role of PRR this might become a therapeutic target in range of diseases.
Diabetic kidney disease and intrarenal RAAS
In DKD, activation of the RAAS in the kidney is a major predictor of progressive renal injury.Citation18 Systemic components of RAAS are down-regulated in diabetes mellitus,Citation19 while there is up-regulation of intra-renal RAAS components as discussed later in this section. Hence, intra-renal RAAS is thought to play a major damaging role. Sustained elevation of intrarenal angiotensin II induces proteinuria and progressive injury of the glomerular endothelium, basement membrane and podocytes.Citation20,Citation21 The locally produced angiotensin II directly induces podocyte injury via activation of AT1 receptors, independent of hemodynamic changes.Citation22 Experimental diabetic animal studies have shown higher,Citation23 lowerCitation24 or unchangedCitation25 kidney angiotensin II levels. In these studies, kidney angiotensin II levels were determined at the onset of diabetes without nephropathy but, when diabetic nephropathy develops and progresses, levels of kidney angiotensin I and II in diabetic rats markedly increases.Citation26 Furthermore, in diabetes plasma renin levels are decreased while pro-renin levels are increased.Citation26,Citation27 It is suggested that intrarenal renin activity increases during the development of diabetic nephropathy and non-proteolytic activation of pro-renin significantly contributes to the increased intrarenal renin activity.Citation26 Increased levels of pro-renin have been shown to predict microvascular complications.Citation26,Citation28 Also, there is a up-regulation of PRR in the kidney of diabetic humansCitation29 and rats.Citation13 Together the combination of increased pro-renin and PRR may create a local environment favorable for PRR activation and for angiotensin II generation. However, in the animal model with overexpression of pro-renin, increased pro-renin was not associated with any cardiac or kidney fibrosis.Citation30 This suggests that the role of PRR in development and progression of kidney disease is not as strong as initially thought.
Microalbuminuria is considered as the first step and a major risk factor in progression of DKD.Citation31 The use of ACEIs and ARBs is a major way for reduction of albuminuria and inhibition of RAAS leading to a reduction in glomerulosclerosis and tubulointerstitial fibrosis and thus is the mainstay for treatment of diabetic nephropathy.Citation32–34 Though ACEIs and ARBs can reduce albuminuria in diabetic patients not all patients respond to the treatment. In the observational study, Joslin Study of the Natural History of Microalbuminuria in Type 1 DiabetesCitation32 microalbuminuria progressed to proteinuria (6.3/100 person-years) in patients who were treated with ACEIs compared to 3.5 per 100 person-years in the absence of treatment. Poor glycemic control and elevated serum cholesterol were found to be the major determinants/predictors of progression. The reasons for lower incidence of progression in the absence of ACEIs treatment were not the subject of this study but, patients who were given ACEIs had higher BP, higher albuminuria, higher cholesterol levels and longer diabetes duration. Along with poor glycemic control and elevated serum cholesterol levels, the incomplete RAAS blockade by ACEIs/ARBs (as discussed later) has also been implicated in the suboptimal response.
In conjunction with the ACE-dependent RAAS activation and potential role of pro-renin, ACE-independent angiotensin II production also plays a crucial role in DKD and our understanding of this pathway has improved based on the recent work. Of the various enzymatic pathways described, serine protease Chymase is the major enzyme implicated in ACE-independent angiotensin II productionCitation35 (). Chymase cleaves angiotensin I at the same site as ACE to form angiotensin II. Chymase is markedly up-regulated in human diabetic kidney particularly in mesangial cells and vascular smooth muscle cells.Citation36 The up-regulation is stronger in hypertensive diabetic kidney disease than those with only diabetic kidney disease.Citation36 In db/db diabetic mice model, ACE inhibition blocks angiotensin-mediated vasoconstriction only in control mice while serine protease inhibition blocks it only in diabetic mice and not vice versa.Citation37 These findings confirm that ACE-independent angiotensin II generation is present and likely contributes equally to the ACE-dependent mechanism in diabetic kidney disease.
Limitations of ACEIs and ARBs
ACEIs block the conversion of angiotensin I to angiotensin II causing decrease in levels of angiotensin II. The decrease in levels of angiotensin II results in loss of feedback inhibition of RAAS and increases renin release from kidney leading to an increase in plasma renin concentration and activity. Increase in plasma renin activity (PRA) increases plasma levels of angiotensin I which is converted to angiotensin II partially restoring the levels via ACE independent pathways; this is known as “ACE Escape”.Citation38 The increased renin can also act through the pro-renin/renin receptor, which may contribute to the renal damage independent of angiotensin II as discussed earlier.
ARBs are selective AT1 receptor blockers which also interrupt the negative feedback control of renin release from the kidneys and lead to an increase in plasma renin concentration and activity along with increased levels of angiotensin I as well as angiotensin II. Angiotensin II may partially stimulate the AT1 receptor and possibly stimulate other receptors like AT2 and AT4, which may be profibrotic and prothromboticCitation39 (). The phenomenon of “Aldosterone Breakthrough” wherein plasma aldosterone levels return to pretreatment levels occurs in 30–40% of patients treated with ACEIs or ARBs. The increased level of aldosterone leads to inflammation, fibrosis and oxidant injury of target organs including kidneys.Citation38,Citation40 Thus, using DRI to block the RAAS pathway at the source of angiotensin I generation would allow a more complete suppression of the angiotensin II activity.
Aliskiren-pharmacokinetics, pharmacodynamics and monitoring of drug effect
Aliskiren is the only available orally effective DRI. It is a non-peptide, low molecular weight drug which was developed by crystal structure analysis and computational molecular modeling. Being a non-peptide molecule, it lacks the extended peptide backbone leading to improved pharmacokinetic properties including better bioavailability, longer half-life and better blood pressure lowering effect. This molecule is highly potent inhibitor of renin with high affinity and specificity for primate renin.Citation41 Aliskiren is available in doses of 150 and 300 mg. Its mean elimination half-life is about 40 h with peak plasma levels in 0.5–3 h after an oral administrationCitation42,Citation43 with steady state achieved in 5–8 days after multiple dosing.Citation43 As monotherapy at doses of 150 mg, it reduces systolic blood pressure (SBP) by 10–15 mm Hg and diastolic blood pressure (DBP) by 2–10 mm Hg (2 mm Hg in 4 weeks study and about 10 mm Hg in 8 weeks study). When used in doses of 300 mg, it leads to a decrease of 12–16 mm Hg of SBP and 5–11 mm Hg of DBP (about 5 mm Hg in 4 weeks study and 11 mm Hg in 8 weeks studies), in sum ambulatory BP decreases by about 10/7 mm Hg.Citation43,Citation44
DRIs bind to catalytic site of renin and inhibit conversion of angiotensinogen to angiotensin I. The proximal site of action of DRIs decrease angiotensin I and angiotensin II levels in a dose-dependent manner. This results in reduction in feedback restriction of renal renin release and increases renin secretion (thus resulting in an increased plasma renin concentration) but the plasma renin activity, the enzymatic activity of renin, is reduced in a dose-dependent manner.Citation45 In total, DRI causes decrease in angiotensin I, angiotensin II and plasma renin activity, which is in contrast to ACEI and ARB and thus gives this agent unique therapeutic potential.
Clinical trials of DRI in diabetic kidney disease
Numerous animal models of DKD have showed significantly improved BP control and proteinuria with the use of aliskiren.Citation46–48 At the level of the glomerulus, use of aliskiren was associated with decreased podocytopathy, glomerular pressure.Citation46,Citation47,Citation50 Tubulointerstitial fibrosis and levels of profibrotic and oxidative markers were found to be reduced with aliskiren.Citation48,Citation49,Citation51 These studies then prompted an investigation of aliskiren in clinical trials.
We searched MEDLINE (PubMed) from 1980 to September 2013. A systematic literature search was performed by using search terms “aliskiren” AND “diabetic nephropathy”. We obtained 89 reference citations and restricted the search to English language (12 references excluded) and humans (18 references excluded). We were left with 59 relevant articles including case reports, observational studies, reviews and randomized controlled studies. Additionally, we performed a manual search of references cited in the selected articles to search for any additional relevant articles. We discussed the findings of the four clinical trials that evaluated the anti-hypertensive and anti-proteinuric effect of aliskiren either alone or in combination of other antihypertensive agents in DKD.
In an open single center prospective exploratory study of 15 patients with type 2 diabetes and microalbuminuria or macroalbuminuria, treatment with aliskiren (300 mg per day) for 28 days was associated with a 44% decrease in albuminuria and a 6–8 mm Hg reduction in SBP compared with baseline values.Citation52 A subsequent cross-over study found that 2 months of treatment with aliskiren (300 mg per day) or irbesartan (300 mg per day) resulted in a similar reduction in albuminuria in 26 patients with hypertension and type 2 diabetes, and that use of the two drugs in combination was associated with a greater decrease in albuminuria than use of either agent alone.Citation53
The aliskiren in the evaluation of proteinuria in diabetes (AVOID) trialCitation54 was a randomized, double-blind, placebo-controlled, multinational study which evaluated the antiproteinuric effect of aliskiren (150 mg force titrated to 300 mg daily after 3 months) in 599 hypertensive patients with type 2 diabetes and nephropathy who were on therapy with losartan at a fixed dose of 100 mg per day. Aliskiren use for 24 weeks was associated with a 20% reduction in albuminuria while no change was noted with placebo. A reduction in urinary albumin: creatinine ratio (UACR) of 50% or more occurred in 25% of patients on aliskiren compared with 12.5% of patients on placebo (p < 0.001). Differences in blood pressure between treatment groups at the end of the study period were small and not significant and changes in albuminuria did not correlate with concomitant changes in arterial blood pressure. Authors concluded that aliskiren might have additional renoprotective effects that are independent of its blood-pressure-lowering effects in patients with hypertension, type 2 diabetes and nephropathy when added to ARB.
In a post hoc analysis of this trial during the 6-month study,Citation55 patients were divided into three groups according to their baseline BP level achieved at the time of randomization, after the 3-month run-in period. The antiproteinuric effect of aliskiren added to standard treatment was equal in different groups. There was statistically significant difference in number of patients with a 50% reduction in UACR from baseline to end of study, between aliskiren and placebo-treated patients across the three BP groups. The change in eGFR was similar between the two treatment groups with the best BP control at baseline but placebo-treated group (BP >/=140/90 mm Hg), had a significantly larger drop in eGFR (5.5 vs. 2.0 mL/min per 1.73 m2 per 6 months). Also, there were more reported symptoms of hypotension among the aliskiren-treated patients in the group with BP < 130/80 as compared with placebo patients, but none required drug discontinuation.
The aliskiren trial in type 2 diabetes using cardio-renal disease endpoints (ALTITUDE) studyCitation56 was a multicenter randomized double-blinded placebo-controlled trial. This was designed to determine whether the addition of aliskiren (300 mg vs. placebo) once daily for 4 years to treatment with ACEI or ARB reduces renal and cardiovascular events in 8600 patients with type 2 diabetes and microalbuminuria, macroalbuminuria or cardiovascular disease. 8561 diabetic patients with either pre-existing renal or cardiovascular disease were randomly assigned to 300 mg/day aliskiren or placebo. At baseline, the majority of patients had nephropathy (59% had an albumin-to-creatinine ratio of 200 mg/g or greater, and 26% had an albumin-to-creatinine ratio between 20 and 199 mg/g; the mean estimated glomerular filtration rate (eGFR) was 57 mL/min per 1.73 m2) and all patients were receiving an ACEI or ARB. After a median follow-up of 32.9 months, more patients in the aliskiren group reached the composite primary end point of ESRD, doubling of serum creatinine, renal death, cardiovascular death, cardiac arrest, heart failure, non-fatal myocardial infarction or non-fatal stroke (18.3 vs. 17.1%). The incidence of renal events (ESRD, renal death or doubling of serum creatinine) was similar with aliskiren and placebo (6 vs. 5.9%). Adverse events requiring cessation of randomized therapy (usually hyperkalemia) were significantly more frequent with aliskiren (13.2 vs. 10.2%). Due to the lack of apparent benefit and higher risk of side effects, the trial was prematurely stopped for futility.
Critical analysis of the evidence for DRI use in DKD
DRI, when used as monotherapy in DKD, clearly have an antihypertensive effect and proteinuria reducing effects that are greater than placebo. These benefits are not superior to those offered by currently used ACEI or ARBs. Thus, DRI is a reasonable alternative to use of ACEI or ARB in DKD, especially in settings where ACEI or ARB may not be used. One such clinical scenario is severe allergic reaction (such as anaphylaxis or angioedema) to ACEI or ARB that precludes further use. As DRI is not known to affect bradykinin level, it would be less likely than ACEI or ARB to cause allergic reaction and in this setting DRI may offer a safer option for RAAS blockade in DKD.Citation57
The use of DRI as dual therapy for RAAS blockade in DKD, that is, DRI with ACEI or ARB is not currently supported by available evidence and may even be harmful. The ALTITUDE trial, as discussed earlier, failed to show additional significant benefit of dual therapy on combined cardiovascular and renal outcomes and the trial had to be stopped early as recommended by the data monitoring committee.Citation56 Significantly more “renal adverse effects” especially hypotension, hyperkalemia and acute kidney injury were observed with dual therapy as compared to ACEI or ARB monotherapy, although the difference in the event rates was small.Citation56 Similar findings were reported in the Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT), a clinical trial of dual RAAS blockade (DRI with ACEI/ARB vs. placebo with ACEI/ARB) in patients with reduced left ventricular ejection fraction hospitalized for congestive heart failure.Citation58 At a 12-month follow-up, no significant difference in cardiovascular outcomes was seen between the two arms, but like the ALTITUDE trial, renal adverse effects were more with the dual therapy. On subgroup analysis, an unexpected finding was that patients with diabetes had worse cardiovascular outcomes on the dual therapy.
Thus, the currently available evidence of using DRI in combination with ACEI or ARB suggests no greater benefit than ACEI or ARB monotherapy in DKD. While the rationale for dual RAAS blockade is to achieve a more complete blockade of RAAS activation at the level of renin and angiotensin II, it is possible that the increased renal adverse effects with combination therapy negate any potential clinical benefit in cardiovascular or renal outcomes. It can be speculated that these effects are due to study design and drug dosages. Patients with DKD or congestive heart failure have different degrees of systemic RAAS activation depending on the amount of organ damage and thus varying degree of disease process may have different responses to combination therapy. Further, confusing this effect is the use of other indicated agents such as beta blockers in congestive heart failure that affect the RAAS pathway independently.Citation59 The ALTITUDE and ASTRONAUT trials did not have run in period to identify tolerance to combination therapy and renal adverse effects. Maximal DRI dose was used in ALTITUDE and ASTRONAUT with unknown but possibly maximal doses of ACEI or ARB that may have thus caused no further benefit and slightly more harm.
Future clinical studies evaluating efficacy of DRI in DKD, especially in combination with ACEI or ARB, should consider the differences in disease characteristics and concurrent medications used in the study arms that may influence outcomes of RAAS blockade. Specifically, a run-in period before the start of the study can help assess if patients can tolerate the combination therapy and exclude those who cannot do so. This has been carefully designed in the Aliskiren Trial of Minimizing Outcomes in Patients with Heart Failure (ATMOSPHERE), a clinical trial comparing aliskiren monotherapy, combination of aliskiren-enalapril therapy and enalapril monotherapy in patients with chronic heart failure.Citation60 The enalapril dose (or equivalent ACEI dose) used in this trial is 20 mg/day which is less than the maximum dose of 40 mg/day. Whether a combination of submaximal doses of DRI and ACEI (or ARB) is better tolerated with clinical benefit needs to be studied.Citation45 Clinical measures to reduce risk of renal adverse events such as ensuring adequate volume status, low potassium diet and avoiding medications that impair potassium excretion or glomerular autoregulation (such as non-steroidal anti-inflammatory drugs) can allow safer use of DRI. Although DRI has been shown to reduce urinary aldosterone in the first three months of use, it would be interesting to study if the phenomenon of aldosterone escape occurs in long-term use of DRI through chymase and other pathways of angiotensin II generation that may make DRI less effective.Citation61 Whether the presence of diabetes nullifies any positive cardiovascular outcome of aliskiren is not totally clear.
Conclusion
Aliskiren is an effective agent that blocks the RAAS pathway. Aliskiren monotherapy has shown promising results in pre-clinical trials and possible renoprotection in early prospective clinical trials. Thus, aliskiren use may be considered in lieu of ACEIs or ARBs. However, current evidence as obtained from randomized clinical trials does not support the use of aliskiren in combination with ACEI or ARB for renoprotection in DKD. Evidence from the ALTITUDE and ASTRONAUT trials suggest no added clinical benefit of DRI with ACEI or ARB in DKD or heart failure respectively and the dual therapy may have slightly more renal adverse effects. Thus, at present, there is little evidence for clinical use of DRI in DKD and its use warrants careful monitoring for hyperkalemia, hypotension or acute kidney injury as with ACEI or ARB therapy.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Collins AJ, Foley RN, Herzog C, et al. US renal data system 2012 annual data report. Am J Kidney Dis. 2013;61(1 Suppl 1):A7, e1–476
- Danser AH, Deinum J. Renin, prorenin and the putative (pro) renin receptor. Hypertension. 2005;46(5):1069–1076
- Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13(Suppl B):9–20
- Matsuba H, Watanabe T, Watanabe M, et al. Immunocytochemical localization of prorenin, renin, and cathepsins B, H, and L in juxtaglomerular cells of rat kidney. J Histochem Cytochem. 1989;37(11):1689–1697
- Jutras I, Reudelhuber TL. Prorenin processing by cathepsin B in vitro and in transfected cells. FEBS Lett. 1999;443(1):48–52
- Mercure C, Lacombe MJ, Khazaie K, Reudelhuber TL. Cathepsin B is not the processing enzyme for mouse prorenin. Am J Physiol Regul Integr Comp Physiol. 2010;298(5):R1212–R1216
- Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59(3):251–287
- Kobori H, Ozawa Y, Suzaki Y, et al. Young Scholars Award Lecture: intratubular angiotensinogen in hypertension and kidney diseases. Am J Hypertens. 2006;19:541–550
- Hayek T, Pavlotzky E, Hamoud S, et al. Tissue angiotensin-converting-enzyme (ACE) deficiency leads to a reduction in oxidative stress and in atherosclerosis: studies in ACE-knockout mice type 2. Arterioscler Thromb Vasc Biol. 2003;23(11):2090–2096
- Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109(11):1417–1427
- Matavelli LC, Huang J, Siragy HM. (Pro)renin receptor contributes to diabetic nephropathy by enhancing renal inflammation. Clin Exp Pharmacol Physiol. 2010;37(3):277–282
- Batenburg WW, Krop M, Garrelds IM, et al. Prorenin is the endogenous agonist of the (pro)renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor. J Hypertens. 2007;25(12):2441–2453
- Huang Y, Wongamorntham S, Kasting J, et al. Renin increases mesangial cell transforming growth factor-beta1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int. 2006;69(1):105–113
- Huang Y, Noble NA, Zhang J, Xu C, Border WA. Renin-stimulated TGF-beta1 expression is regulated by a mitogen-activated protein kinase in mesangial cells. Kidney Int. 2007;72(1):45–52
- Nguyen G. Renin, (pro)renin and receptor: an update. Clin Sci (Lond). 2011;120(5):169–178
- Advani A, Kelly DJ, Cox AJ, et al. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H + -ATPase in the kidney. Hypertension. 2009;54(2):261–269
- Kinouchi K, Ichihara A, Sano M, et al. The (pro)renin receptor/ATP6AP2 is essential for vacuolar H + -ATPase assembly in murine cardiomyocytes. Circ Res. 2010;107(1):30–34
- Carey RM, Siragy HM. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol Metab. 2003;14(6):274–281
- Price DA, Porter LE, Gordon M, et al. The paradox of the low-renin state in diabetic nephropathy. J Am Soc Nephrol. 1999;10(11):2382–2391
- Hoffmann S, Podlich D, Hähnel B, Kriz W, Gretz N. Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol. 2004;15(6):1475–1487
- Whaley-Connell AT, Chowdhury NA, Hayden MR, et al. Oxidative stress and glomerular filtration barrier injury: role of the renin-angiotensin system in the Ren2 transgenic rat. Am J Physiol Renal Physiol. 2006;291(6):F1308–F1314
- Durvasula RV, Petermann AT, Hiromura K, et al. Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int. 2004;65(1):30–39
- Zimpelmann J, Kumar D, Levine DZ, et al. Early diabetes mellitus stimulates proximal tubule renin mRNA expression in the rat. Kidney Int. 2000;58(6):2320–2330
- Vallon V, Wead LM, Blantz RC. Renal hemodynamics and plasma and kidney angiotensin II in established diabetes mellitus in rats: effect of sodium and salt restriction. J Am Soc Nephrol. 1995;5(10):1761–1767
- Campbell DJ, Kelly DJ, Wilkinson-Berka JL, Cooper ME, Skinner SL. Increased bradykinin and “normal” angiotensin peptide levels in diabetic Sprague-Dawley and transgenic (mRen-2)27 rats. Kidney Int. 1999;56(1):211–221
- Ichihara A, Hayashi M, Kaneshiro Y, et al. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest. 2004;114(8):1128–1135
- Luetscher JA, Kraemer FB, Wilson DM, Schwartz HC, Bryer-Ash M. Increased plasma inactive renin in diabetes mellitus. A marker of microvascular complications. N Engl J Med. 1985;312(22):1412–1417
- Deinum J, Rønn B, Mathiesen E, Derkx FH, Hop WC, Schalekamp MA. Increase in serum prorenin precedes onset of microalbuminuria in patients with insulin-dependent diabetes mellitus. Diabetologia. 1999;42(8):1006–1010. Erratum in: Diabetologia. 1999;42(12):1444
- Takahashi K, Yamamoto H, Hirose T, et al. Expression of (pro)renin receptor in human kidneys with end-stage kidney disease due to diabetic nephropathy. Peptides. 2010;31(7):1405–1408
- Mercure C, Prescott G, Lacombe MJ, Silversides DW, Reudelhuber TL. Chronic increases in circulating prorenin are not associated with renal or cardiac pathologies. Hypertension. 2009;53(6):1062–1069
- Perkins BA, Ficociello LH, Ostrander BE, et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18(4):1353–1361
- Ficociello LH, Perkins BA, Silva KH, et al. Determinants of progression from microalbuminuria to proteinuria in patients who have type 1 diabetes and are treated with angiotensin-converting enzyme inhibitors. Clin J Am Soc Nephrol. 2007;2(3):461–469
- Brenner BM, Cooper ME, de Zeeuw D, et al. RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869
- Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P. Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870–878
- Dell'Italia LJ, Husain A. Dissecting the role of chymase in angiotensin II formation and heart and blood vessel diseases. Curr Opin Cardiol. 2002;17(4):374–379
- Huang XR, Chen WY, Truong LD, Lan HY. Chymase is upregulated in diabetic nephropathy: implications for an alternative pathway of angiotensin II-mediated diabetic renal and vascular disease. J Am Soc Nephrol. 2003;14(7):1738–1747
- Park S, Bivona BJ, Kobori H, et al. Major role for ACE-independent intrarenal ANG II formation in type II diabetes. Am J Physiol Renal Physiol. 2010;298(1):F37–F48
- Rashid HU, Mende C. The role of direct renin inhibition in clinical practice focus on combination therapy. Am J Cardiovasc Drugs. 2011;11(5):303–315
- Strauss MH, Hall AS. Angiotensin receptor blockers may increase risk of myocardial infarction: unraveling the ARB-MI paradox. Circulation. 2006;114(8):838–854. Erratum in: Circulation. 2006;114(19):e576
- Schrier RW. Aldosterone ‘escape’ vs ‘breakthrough’. Nat Rev Nephrol. 2010;6(2):61
- Wood JM, Maibaum J, Rahuel J, et al. Structure-based design of aliskiren, a novel orally effective renin inhibitor. Biochem Biophys Res Commun. 2003;308(4):698–705
- Nussberger J, Wuerzner G, Jensen C, Brunner HR. Angiotensin II suppression in humans by the orally active renin inhibitor Aliskiren (SPP100): comparison with enalapril. Hypertension. 2002;39(1):E1–E8
- Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomized, double-blind trial. Lancet. 2007;370(9583):221–229. Erratum in: Lancet. 2007;370(9598):1542
- Gradman AH, Schmieder RE, Lins RL, Nussberger J, Chiang Y, Bedigian MP. Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients. Circulation. 2005;111(8):1012–1018
- Azizi M, Ménard J, Bissery A, et al. Hormonal and hemodynamic effects of aliskiren and valsartan and their combination in sodium-replete normotensive individuals. Clin J Am Soc Nephrol 2007;2:947–955
- Whaley-Connell A, Nistala R, Habibi J, et al. Comparative effect of direct renin inhibition and AT1R blockade on glomerular filtration barrier injury in the transgenic Ren2 rat. Am J Physiol Renal Physiol. 2010;298(3):F655–F661
- Vanourková Z, Kramer HJ, Husková Z, Cervenka L, Vanecková I. Despite similar reduction of blood pressure and renal ANG II and ET-1 levels aliskiren but not losartan normalizes albuminuria in hypertensive Ren-2 rats. Physiol Res. 2010;59(3):339–345
- Rakusan D, Kujal P, Kramer HJ, et al. Persistent antihypertensive effect of aliskiren is accompanied by reduced proteinuria and normalization of glomerular area in Ren-2 transgenic rats. Am J Physiol Renal Physiol. 2010;299(4):F758–F766
- Pilz B, Shagdarsuren E, Wellner M, et al. Aliskiren, a human renin inhibitor, ameliorates cardiac and renal damage in double-transgenic rats. Hypertension. 2005;46(3):569–576
- Dong YF, Liu L, Lai ZF, et al. Aliskiren enhances protective effects of valsartan against type 2 diabetic nephropathy in mice. J Hypertens. 2010;28(7):1554–1565
- Kelly DJ, Zhang Y, Moe G, Naik G, Gilbert RE. Aliskiren, a novel renin inhibitor, is renoprotective in a model of advanced diabetic nephropathy in rats. Diabetologia. 2007;50(11):2398–2404
- Persson F, Rossing P, Schjoedt KJ, et al. Time course of the antiproteinuric and antihypertensive effects of direct renin inhibition in type 2 diabetes. Kidney Int. 2008;73(12):1419–1425
- Persson F, Rossing P, Reinhard H, et al. Renal effects of aliskiren compared with and in combination with irbesartan in patients with type 2 diabetes, hypertension, and albuminuria. Diabetes Care. 2009;32(10):1873–1879
- Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. AVOID Study Investigators. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358(23):2433–2446
- Persson F, Lewis JB, Lewis EJ, Rossing P, Hollenberg NK, Parving HH. Aliskiren in combination with losartan reduces albuminuria independent of baseline blood pressure in patients with type 2 diabetes and nephropathy. Clin J Am Soc Nephrol. 2011;6(5):1025–1031
- Parving HH, Brenner BM, McMurray JJ, et al. ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367(23):2204–2213
- Anzaldua DA, Schmitz PG. Aliskiren as an alternative in a patient with life-threatening ACE inhibitor-induced angioedema. Am J Kidney Dis. 2008;51(3):532–533
- Gheorghiade M, Böhm M, Greene SJ, et al. ASTRONAUT Investigators and Coordinators. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA. 2013;309(11):1125–1135
- Holmer SR, Hense HW, Danser AH, Mayer B, Riegger GA, Schunkert H. Beta adrenergic blockers lower renin in patients treated with ACE inhibitors and diuretics. Heart. 1998;80(1):45–48
- McMurray JJ, Abraham WT, Dickstein K, Køber L, Massie BM, Krum H. Aliskiren, ALTITUDE, and the implications for ATMOSPHERE. Eur J Heart Fail. 2012;14(4):341–343
- Persson F, Lewis JB, Lewis EJ, Rossing P, Hollenberg NK, Hans-Henrik P. Impact of aliskiren treatment on urinary aldosterone levels in patients with type 2 diabetes and nephropathy: an AVOID substudy. J Renin Angiotensin Aldosterone Syst. 2012;13(1):118–121