Abstract
Relationship between methylenetetrahydrofolate reductase (MTHFR) A1298C gene polymorphism and type 2 diabetic nephropathy (T2DN) risk is still unclear. This study was performed to evaluate if there is an association between the MTHFR A1298C gene polymorphism and T2DN risk using meta-analysis. The relevant reports were searched and identified from PubMed, Cochrane Library on 1 October 2013, and eligible studies were included and synthesized. Eight reports were recruited into this meta-analysis for the association of the MTHFR A1298C gene polymorphism with T2DN risk. The MTHFR A1298C C allele or CC genotype was shown to be not associated with T2DN risk (C allele: OR = 0.76, 95% CI: 0.43–1.34, p = 0.34; CC genotype: OR = 1.18, 95% CI: 0.63–2.22, p = 0.60). Interestingly, AA genotype was associated with the T2DN risk (OR = 0.68, 95% CI: 0.49–0.96, p = 0.03). In the sensitivity analysis according to the Hardy–Weinberg equilibrium (HWE), the results were consistent with those in non-sensitivity analysis. However, in the sensitivity analysis according to the control source from hospital, sample size of case (≥100), sample size of case (<100), the MTHFR A1298C gene polymorphism was not associated with T2DN risk. In conclusion, the MTHFR A1298C gene polymorphism was not associated with T2DN risk. However, additional studies are required to firmly establish a correlation between the MTHFR A1298C gene polymorphism and T2DN risk.
Introduction
Methylenetetrahydrofolate reductase (MTHFR) is a critical enzyme in folate metabolism and is involved in DNA synthesis, DNA repair and DNA methylation.Citation1 MTHFR is involved in the one-carbon cycle, which is of importance for nucleotide synthesis and methylation of DNA, membranes, proteins and lipids; and the MTHFR gene includes two common polymorphisms (rs1801133 or C677T; rs1801131 or A1298C), both of which alter the enzyme activity.Citation2 MTHFR is a key enzyme in folate and homocysteine metabolism, and MTHFR A1298C polymorphism with the folate metabolism.Citation3 Deficiency of MTHFR may be associated with the levels of plasma folate and homocysteine metabolism, which in turn is associated with an increased risk of vascular diseases including the predisposition to nephropathy among diabetic patients.Citation4 The present evidence indicates that A1298C polymorphism is associated with the risk of type 2 diabetic nephropathy (T2DN).Citation3
Diabetic nephropathy (DN) is one of the most serious microvascular complications of diabetes mellitus, and is the primary cause of end-stage renal disease (ESRD) worldwide.Citation5 Susceptibility to DN has an inherent genetic basis as evidenced by familial aggregation and ethnic-specific prevalence rates, and DN is a complex disease and is caused by both environmental and genetic factors.Citation6,Citation7 Type 2 diabetic nephropathy (T2DN) is one of the most important types of DN and is increasing in prevalence. T2DN is associated with high global levels of mortality and morbidity associated with increased morbidity and mortality among diabetic patients, and ESRD due to T2DN is a very common condition.Citation8
Gene polymorphism factor has been shown to affect protein activity in various diseases associated with the development of degenerate cellular mechanisms.Citation9 The epidemiological evidence on the association of the MTHFR A1298C gene polymorphism and T2DN from investigations performed over the past few decades is weak, predominantly due to sparseness of data or disagreements among the reported investigations. Evidence from meta-analysis might provide more insight into the aforementioned association and proved to be more significant compared with data from individual investigations. The current meta-analysis was performed to investigate whether the MTHFR A1298C gene polymorphism associated with the risk of developing T2DN.
Materials and methods
Search strategy for the association of MTHFR A1298C gene polymorphism with the risk of T2DN
Relevant studies were extracted from the electronic databases of PubMed, Cochrane Library on 1 October 2013. The retrieval strings entered into these databases were: “(methylenetetrahydrofolate reductase OR MTHFR) AND (diabetic nephropathy OR DN OR diabetes mellitus nephropathy) AND (gene OR polymorphism OR allele OR genotype OR variant OR variation OR mutation) AND A1298C”. Additional reports were identified by scrutinizing the references cited in the recruited articles.
Inclusion and exclusion criteria
Inclusion criteria
(1) There had to be at least two comparison groups (case group vs. control group); (2) the investigation should provide data on MTHFR A1298C genotype distribution.
Exclusion criteria
(1) Preliminary results not on MTHFR A1298C gene polymorphism or outcome; (2) investigation of the role of MTHFR gene expression related to disease; (3) if multiple publications for the same data from the same study group occurred, we only recruited the later dated paper into our final analysis.
Data extraction and synthesis
The following information from each eligible study was extracted independently by two investigators: first author’s surname, year of publication, country, ethnicity, genotyping methods, control source of the control group and the number of cases and controls for MTHFR A1298C genotypes. The results were compared and disagreement was resolved by discussion.
Statistical analysis
Cochrane Review Manager Version 5 (Cochrane Library, Exeter, UK) was used to calculate the available data from each study. The pooled statistics were counted using the fixed effects model, but a random effects model was used when the p value of the heterogeneity test was less than 0.1.Citation10,Citation11 Results were expressed with odds ratios (OR) for dichotomous data, and 95% confidence intervals (CI) were also calculated. p < 0.05 was required for the pooled OR to be statistically significant.Citation12,Citation13 I2 was used to test the heterogeneity among the included studies.Citation9,Citation14 A Chi-square test using a web-based program was applied to determine if genotype distribution of the control population reported for MTHFR A1298C conformed to th HWE (p < 0.05 was considered significant). Sensitivity analysis was performed if HWE disequilibrium existed. Sensitivity analysis was also performed according to the source of the controls (population vs. hospital), and sample size of case or control (<100 vs. ≥100).
Results
Study characteristics
Eight studiesCitation4,Citation15–21 reporting the relationship between MTHFR A1298C gene polymorphism and T2DN susceptibility were recruited into this meta-analysis. The data of our interest were extracted () and the included investigations contained 1387 patients with T2DN and 1533 controls. The average distribution frequency of the MTHFR A1298C C allele in the T2DN group was 31.42% and the average frequency in the control group was 39.43%. The average distribution frequency of MTHFR C allele in the case group was slightly lower when compared to the control group (case/control = 0.80). In the sub-group analysis, the average distributions of MTHFR A1298C C allele frequency in Caucasians were 32.12% in cases and 43.81% for controls. Furthermore, the distribution frequencies of MTHFR C allele in Africans were 27.94% in cases and 17.50% for controls. The value of ratio of case/control for the average distribution frequency of MTHFR A1298C C allele in Caucasians was less than 1, but the Africans’ was more than 1 (Caucasians: case/control = 0.73; Africans: case/control = 1.60).
Table 1. General characteristics of the included studies in this meta-analysis.
Association of MTHFR A1298C gene polymorphism with T2DN susceptibility
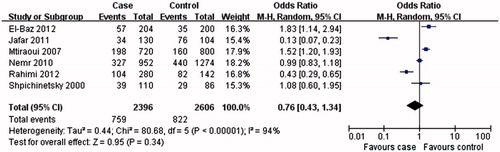
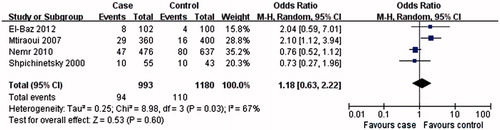
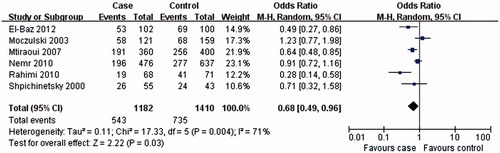
The results of this meta-analysis showed that MTHFR A1298C C allele or CC genotype was not associated with T2DN susceptibility (C allele: OR = 0.76, 95% CI: 0.43–1.34, p = 0.34; CC genotype: OR = 1.18, 95% CI: 0.63–2.22, p = 0.60; and ; ). Interestingly, AA genotype was associated with the T2DN risk (OR = 0.68, 95% CI: 0.49–0.96, p = 0.03; and ).
Table 2. Meta-analysis of the association of MTHFR A1298C gene polymorphism with T2DN risk.
Sensitivity analysis
Sensitivity analysis according to genotype distribution of the control population for MTHFR A1298C gene polymorphism conformed to HWE for the relationship between MTHFR A1298C gene polymorphism and T2DN risk. The genotype distribution of the control population in three studiesCitation4,Citation15,Citation16 was conform to HWE. Overall, the results from sensitivity analysis according to HWE were similar to those from non-sensitivity analysis, and MTHFR A1298C C allele or CC genotype was not associated with T2DN susceptibility ().
Further, sensitivity assessment based on the source of the controls (healthy vs. hospital) was also conducted. In the sensitivity analysis according to the source of the controls from hospital; the results of the relationship between MTHFR C allele or CC genotype and T2DN risk were similar to the non-sensitivity analysis, but not the AA genotype (). In the sensitivity analysis, according to the source of the controls from healthy, the results of the relationship between MTHFR C allele or AA genotype and T2DN risk were similar to the non-sensitivity analysis, but not the CC genotype ().
Sensitivity analysis for the relationship between the MTHFR A1298C gene polymorphism and T2DN risk according to sample size of case (<100 vs. ≥100) was additionally performed. The results of C allele or CC genotype in the sensitivity analysis (≥100) were similar as the non-sensitivity analysis, and C allele or CC genotype was not associated with T2DN susceptibility (). However, AA genotype was not associated with T2DN susceptibility in the sensitivity analysis according to sample size of case (<100 vs. ≥100) ().
Discussion
Our results demonstrated that MTHFR A1298C C allele or CC genotype was not associated with T2DN risk, but the AA genotype might be a protective factor for T2DN disease. The results of MTHFR A1298C C allele or CC genotype from most of the sensitivity analyses were similar with those of non-sensitivity analysis, but not the sensitivity analysis according to the control source from population. However, there was only one included study for the sensitivity analysis according to the control source from population. The results for sensitivity analysis according to the control source from population might be less robust. It might be safe to draw a conclusion that MTHFR A1298C C allele or CC genotype was not associated with T2DN risk.
Furthermore, MTHFR AA genotype was associated with a reduced risk of T2DN in our meta-analysis. In the sensitivity analysis according to HWE and the control source from population, the result for AA genotype was similar to that in non-sensitivity analysis. However, in the sensitivity analysis according to the control source from hospital, sensitivity analysis according to sample size of case (≥100), sensitivity analysis according to sample size of case (<100), this association was not found. The number of included studies was a little small. More studies on the relationship between MTHFR A1298C AA genotype and T2DN risk should be performed in the future.
The average distribution frequency of the MTHFR A1298C C allele in cases showed a 0.80-fold increase compared with the control group. Consequently, C allele might be a protective factor for T2DN disease. In this meta-analysis, we also found that the C allele might be a protective factor for T2DN disease, but this was not statistically different. Furthermore, ratio of case/control for the average distribution frequency of MTHFR A1298C C allele in Caucasians was less than that in the Africans. The distribution of MTHFR A1298C gene polymorphism between Caucasian population and African population was not imbalanced. Because there was only one report from Africans and others were from Caucasians, the sub-group by ethnicity was not performed. More investigation on the MTHFR A1298C gene polymorphism among different races should be performed in the future.
There was no other meta-analysis performed to assess the relationship between MTHFR A1298C gene polymorphism and T2DN risk. Chang et al.Citation5 conducted a meta-analysis to investigate the role of MTHFR C677T polymorphism on DN in Chinese type 2 diabetic patients, and reported that MTHFR C677T polymorphism might influence DN risk in the Chinese population.
Even though meta-analyses aim to combine comparable studies, to increase sample size and statistical significance, and identify patterns in various studies, the quality of such analyses might be limited by publication bias, the sampling method, variations in genetic background of the subjects, and differences in the used protocols. We aimed to minimize these limitations by using appropriate inclusion and exclusion criteria to reduce selection bias, tested the HWE for distribution of the genotypes to eliminate different genetic backgrounds among the participants. Overall, the results from our study support the notion that MTHFR A1298C C allele or CC genotype may not be considered as a risk factor for T2DN risk. However, more studies should be performed in the future.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Sameer AS, Shah ZA, Nissar S, Mudassar S, Siddiqi MA. Risk of colorectal cancer associated with the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in the Kashmiri population. Genet Mol Res. 2011;10(2):1200–1210
- Saetre P, Vares M, Werge T, et al. Methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C polymorphisms and age of onset in schizophrenia: a combined analysis of independent samples. Am J Med Genet B Neuropsychiatr Genet. 2011;156(2):215–224
- Wu X, Wang X, Chan Y, Jia S, Luo Y, Tang W. Folate metabolism gene polymorphisms MTHFR C677T and A1298C and risk for Down syndrome offspring: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2013;167(2):154–159
- El-Baz R, Settin A, Ismaeel A, et al. MTHFR C677T, A1298C and ACE I/D polymorphisms as risk factors for diabetic nephropathy among type 2 diabetic patients. J Renin Angiotensin Aldosterone Syst. 2012;13(4):472–477
- Chang WW, Zhang L, Yao YS, Su H, Jin YL, Chen Y. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and susceptibility to diabetic nephropathy in Chinese type 2 diabetic patients: a meta-analysis. Ren Fail. 2013;35(7):1038–1043
- Mooyaart AL. Genetic associations in diabetic nephropathy. Clin Exp Nephrol. 2013. [Epub ahead of print]. doi: 10.1007/s10157-013-0874-9
- Zhou TB, Xu HL, Yin SS. Association between endothelial nitric oxide synthase Glu298Asp gene polymorphism and diabetic nephropathy susceptibility. Ren Fail. 2013;35(1):173–178
- Khajehdehi P, Pakfetrat M, Javidnia K, et al. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-beta and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double-blind and placebo-controlled study. Scand J Urol Nephrol. 2011;45(5):365–370
- Zhou TB, Yin SS, Jiang ZP. Association of angiotensin II type-1 receptor A1166C gene polymorphism with the susceptibility of end-stage renal disease. J Recept Signal Transduct Res. 2013;33(5):325–331
- Zhou TB, Yin SS. Association of matrix metalloproteinase-9 level with the risk of renal involvement for Henoch-Schonlein purpura in children. Ren Fail. 2013;35(3):425–429
- Zhou TB, Yin SS, Liang R. A meta-analysis of the association between angiotensin-converting enzyme insertion/deletion gene polymorphism and end-stage renal disease risk in IgA nephropathy patients. J Renin Angiotensin Aldosterone Syst. 2013;14(3):235–241
- Cheng HY, You HY, Zhou TB. Relationship between GSTM1/GSTT1 null genotypes and renal cell carcinoma risk: a meta-analysis. Ren Fail. 2012;34(8):1052–1057
- Zhou TB, Qin YH, Xu HL. Association of apoE gene expression and its gene polymorphism with nephrotic syndrome susceptibility: a meta-analysis of experimental and human studies. Mol Biol Rep. 2012;39(10):9347–9354
- Zhou TB, Yin SS. Association of endothelial nitric oxide synthase Glu298Asp gene polymorphism with the risk of end-stage renal disease. Ren Fail. 2013;35(4):573–578
- Shpichinetsky V, Raz I, Friedlander Y, et al. The association between two common mutations C677T and A1298C in human methylenetetrahydrofolate reductase gene and the risk for diabetic nephropathy in type II diabetic patients. J Nutr. 2000;130(10):2493–2497
- Mtiraoui N, Ezzidi I, Chaieb M, et al. MTHFR C677T and A1298C gene polymorphisms and hyperhomocysteinemia as risk factors of diabetic nephropathy in type 2 diabetes patients. Diabetes Res Clin Pract. 2007;75(1):99–106
- Nemr R, Salman RA, Jawad LH, Juma EA, Keleshian SH, Almawi WY. Differential contribution of MTHFR C677T variant to the risk of diabetic nephropathy in Lebanese and Bahraini Arabs. Clin Chem Lab Med. 2010;48(8):1091–1094
- Rahimi M, Hasanvand A, Rahimi Z, et al. Synergistic effects of the MTHFR C677T and A1298C polymorphisms on the increased risk of micro- and macro-albuminuria and progression of diabetic nephropathy among Iranians with type 2 diabetes mellitus. Clin Biochem. 2010;43(16–17):1333–1339
- Moczulski D, Fojcik H, Zukowska-Szczechowska E, Szydlowska I, Grzeszczak W. Effects of the C677T and A1298C polymorphisms of the MTHFR gene on the genetic predisposition for diabetic nephropathy. Nephrol Dial Transplant. 2003;18(8):1535–1540
- Rahimi Z, Hasanvand A, Felehgari V. Interaction of MTHFR 1298C with ACE D allele augments the risk of diabetic nephropathy in Western Iran. DNA Cell Biol. 2012;31(4):553–559
- Jafari Y, Rahimi Z, Vaisi-Raygani A, Rezaei M. Interaction of eNOS polymorphism with MTHFR variants increase the risk of diabetic nephropathy and its progression in type 2 diabetes mellitus patients. Mol Cell Biochem. 2011;353(1–2):23–34