Abstract
Background and objective: Endothelial nitric oxide synthase (eNOS) is one of the potent regulators of intra renal hemodynamics. Polymorphisms of eNOS gene may be involved in the progression of renal disease, and may be the causative factors that contribute to the deterioration of renal functions. During the past decades, several studies investigated the association of eNOS polymorphisms with the risk of end-stage renal disease (ESRD), but the results remain unclear and the mechanisms are not defined. Our study was designed to examine the role of different eNOS genetic polymorphisms in the progression of ESRD. Materials and methods: Relevant studies were identified through PubMed, Embase, Medline and CNKI (China National Knowledge Infrastructure) database published between January 2000 and November 2013. The association between eNOS polymorphisms and ESRD susceptibility was assessed by pooled odds ratios (ORs) and 95% confidence intervals (95% CI) in fixed or random effects models. Results: Sixteen articles were identified for the analysis of association between eNOS gene polymorphisms and ESRD risk. A total of 2729 patients and 2190 controls for 4b/a, 851 patients and 1171 controls for G894T, and 513 patients and 487 controls for T786C were included in our analysis. Overall, 4a allele of 4b/a polymorphism produced a significant association in the global population (OR = 1.47, 95% CI = 1.05–2.06, p = 0.03) in a random-effect model; T allele of G894T was also significantly associated with ESRD susceptibility in overall populations (OR = 2.12, 95% CI = 1.44–3.12, p = 0.0001). Furthermore, 4a and T carriers were significantly associated with ESRD risk as well. No association was found between T786C polymorphism and ESRD. Conclusion: The evidence accumulated suggested that 4b/a and G894T polymorphisms in the eNOS gene were associated with ESRD susceptibility, indicating that 4a and T allele carriers might become significant genetic molecular markers for the onset of ESRD in overall populations. However, more studies should be performed in the further studies.
Introduction
End-stage renal disease (ESRD) represents the final stage of chronic kidney disease. It is a worldwide health problem with an irreversible loss of endogenous renal function and with an epidemic extent.Citation1,Citation2 Patients with ESRD experience high rates of morbidity and mortality.Citation3 Studies have showed that incidence and prevalence counts of ESRD in the United States are expected to increase by 44 and 85% from 2000 to 2015, respectively.Citation4 ESRD is a multifactorial disease. Prior studies have identified that proteinuria, calciphylaxis, older age, elevated blood pressure and elevated serum creatinine level may be the independent risk factors for ESRD.Citation5 However, there was rare genetic molecular marker to predict the onset of ESRD. Thus, knowing various genetic factors may be helpful to understand the pathophysiology of the ESRD.
Many genes along with environmental factors may make an individual susceptible for developing ESRD. More recent studies have established endothelial nitric oxide synthase (eNOS) gene polymorphisms as genetic molecular marker for the onset of ESRD in overall populations.Citation6 NOS was first recognized and reported in 1989,Citation7,Citation8 including three NOS isoforms (NOS-1, NOS-2 and NOS-3) that are products of different genes, with different localization, regulation, catalytic properties and inhibitor sensitivity. NOS-3, also called eNOS, is found and mostly expressed in vascular endothelial cells.Citation9 It can inhibit platelet aggregation, leucocyte adhesion and vascular inflammation, control vascular smooth muscle proliferation, stimulate angiogenesis and activate endothelial progenitor cells.Citation10 Three eNOS polymorphisms, the Intron 4 27-bp repeat (4b/a), the G894T (Glu298Asp) missense mutation in exon 7 and the T786C single-nucleotide polymorphism in the promoter region have been studied. T786C reduces eNOS transcription;Citation11 G894T may be associated with a decrease of eNOS activityCitation12 and 4b/a polymorphism has been reported to be associated with decreased plasma nitric oxide (NO) concentrations that may reflect the activity of eNOS.Citation13
These polymorphisms were all reported to be associated with nephropathy. However, the association between ESRD risk and polymorphisms found in eNOS is still controversial. Some studies have found that different genotypes of eNOS were associated with ESRD,Citation14 whereas other reports suggest that they may not be significantly associated with ESRD risk.Citation15 It could be every single study is inadequate in achieving a comprehensive and reliable conclusion. To better address the association between eNOS polymorphisms and ESRD risk, we performed a meta-analysis from all eligible studies based on samples of different ethnicities.
Materials and methods
Search strategy and eligibility criteria
A comprehensive search was conducted using the following electronic databases PubMed, Embase, Medline and China National Knowledge Infrastructure (CNKI) for related reports published between January 2000 and November 2013. Articles reporting the association between ESRD and eNOS polymorphisms were identified. The Medical Subject Heading (MeSH) terms “endothelial nitric oxide synthase gene”, “eNOS”, “polymorphism” and “end-stage renal disease” were employed as the searching words. All studies matching the eligibility criteria were retrieved, and references were checked for other relevant publications.
Criteria for article screening
Studies included had to meet all the following criteria: (1) case-control or cohort association studies included at least one of the three polymorphisms, 4b/a, G894T, T786C; (2) based on unrelated individuals; (3) the outcome had to be ESRD; (4) when the same authors reported two or more publications on possibly the same patient populations, only the most recent or complete study was included into this meta-analysis; and (5) the genotype distribution of the control population must be in the Hardy–Weinberg equilibrium (HWE).
Data extraction
Two investigators independently extracted the data and reached a consensus on all of the items. Any disagreement was resolved by discussing with the third expert. Data retrieved from the reports included author, publication year, ethnicity, age, sample size and type of sample for genotyping. Genotype distribution in both cases and controls were also obtained.
Statistical analysis
The pooled odd ratio (OR) with 95% confidence intervals (95% CI) was used to assess the strength of association between eNOS polymorphisms and ESRD. The significance of the pooled OR was determined by the Z-test, and a p value less than 0.05 was considered significant. This study examined the association between eNOS and ESRD risk compared with that for per-allele model (4a vs. 4b for 4b/a, T vs. G for G894T, C vs. T for T786C), dominant model (4aa + 4ba vs. 4bb for 4b/a, TT + GT vs. GG for G894T, CC + TC vs. TT for T786C), recessive model (4aa vs. 4ba + 4bb for 4b/a, TT vs. GT + GG for G894T, CC vs. TC + TT for T786C). The heterogeneity for the included articles was evaluated using Cochran’s Q test and I2 statistics. p Value less than 0.1 and I2 less than 50% were considered to be statistically significant. When a significant heterogeneity existed across the included studies (I2 > 50%), the random effect model was used; when there was no significant heterogeneity across the included studies (I2 < 50%), the fixed effect model was used. To assess whether our results were substantially influenced by the presence of any individual study, we conducted a sensitivity analysis by systematically removing each study and recalculating the significance of the result. Begg’s funnel plot was performed to examine the publication bias. Analyses were carried out using the Review manager 5.2 (The Cochrane Collaboration). All tests were two-sided.
Results
Characteristics of studies included
With our search criterion, 36 papers were found. Twenty-two papers concerning association research between eNOS polymorphisms and ESRD were identified. Among those 22 papers, 16 papers met our criteria and six papers were excluded for they did not provide detailed genotype information or a lack of control group data. Of the 16 include studies, two were from China,Citation16,Citation17 two were from Brazil,Citation18,Citation19 one was from France,Citation20 one was from India,Citation21 five were from Japan,Citation22–26 one was from Poland,Citation27 one was from Greece and CyprusCitation28 one was from Egypt,Citation29 one was from CanadaCitation30 and one was from Hungary.Citation31 The major characteristics of the 16 eligible publications are reported in . The association of eNOS gene polymorphisms in ESRD risk was shown in .
Table 1. Characteristics of the studies evaluating the effects of eNOS gene polymorphism on ESRD risk.
Table 2. Meta-analysis of eNOS gene polymorphisms in ESRD.
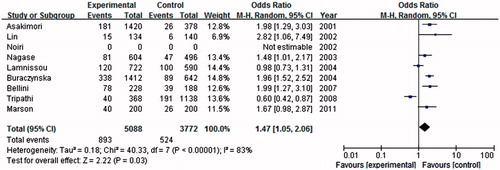
Association of the eNOS 4b/a gene polymorphism with ESRD risk
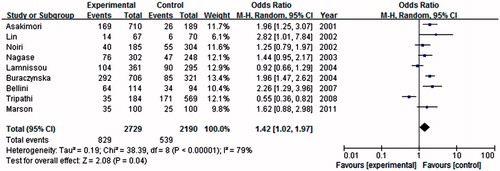
In this meta-analysis, nine studies were considered eNOS 4b/a gene polymorphisms with ESRD risk, including 2729 cases and 2190 controls. As shown in , we found that eNOS 4a allele was associated with an increased risk of ESRD when compared with the 4b allele in global populations (OR = 1.47, 95% CI = 1.05–2.06, p = 0.03) in a random-effect model. Under dominant model, a similar significant result between 4a genotype (4aa + 4ba) and the risk of ESRD in comparison with eNOS 4bb (OR = 1.42, 95% CI = 1.02–1.97, p = 0.04) in a random-effect model (). Under the recessive genetic model, 4aa genotype was significantly associated with an increased risk of ESRD in the global populations under the homozygote genetic model (OR = 2.39, 95% CI = 1.59–3.61, p < 0.0001) in a fixed model.
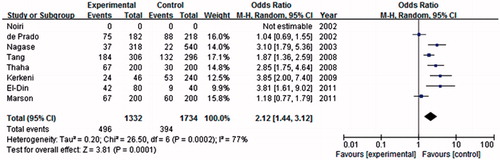
Association of the eNOS-894 polymorphism with ESRD risk
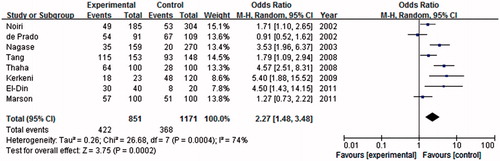
A total of eight studies were included in association investigation of eNOS-894 polymorphism and ESRD risk. There were 851 patients and 1171 controls. The result showed a weak association between the T allele and ESRD risk compared with the G allele in the global population (OR = 2.12, 95% CI = 1.44–3.12, p = 0.0001) in the random-effect model (). Similar association was found in the dominant model (OR = 2.27, 95% CI = 1.48–3.48, p = 0.0002). As shown in , significant association between eNOS-894 TT and the risk of ESRD was found in the global population in the recessive genetic model (OR = 2.00, 95% CI = 1.44–2.78, p < 0.0001).
Association of the eNOS-789 polymorphisms with ESRD risk
Only three studies were included in association investigation of eNOS-786 polymorphism and ESRD risk. As shown in , there was no association between the C allele and ESRD risk compared with the T allele in the global population (OR = 1.23, 95% CI = 0.74–2.05, p = 0.42). No association betweene NOS-786 CC and the risk of ESRD was found in the global population in the dominant model (OR = 1.22, 95% CI = 0.69–2.15, p = 0.49) and in the recessive genetic model (OR = 1.06, 95% CI = 0.69–1.63, p = 0.78).
Sensitivity analyses and publication bias
A single study included in the meta-analysis was deleted each time to reflect the influence of the individual data set to the pooled ORs. For 4b/4a polymorphism, if Lamnissou’s and Tripathi’s studies were excluded, no heterogeneity was found between each included studies (I2 = 0), and the corresponding pooled ORs were not materially changed. This procedure confirmed the stability of our overall result.
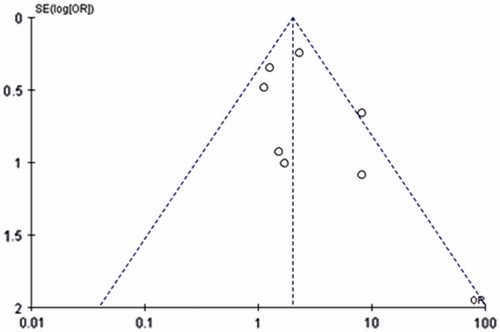
Egger’s test was conducted to assess the publication bias of the literature. The shape of funnel plots did not reveal any evidence of funnel plot asymmetry. As shown in , the statistical results still did not show publication bias.
Discussion
ESRD is a major health problem associated with very high morbidity and mortality. Reducing the incidence of ESRD is widely recognized as a major public health goal. Recently, many potential risk factors have been suggested, such as angiotensin converting enzyme (ACE), uncoupling protein (UCP2), eNOS.Citation32–34 However, data on the risk factors for the pathogenesis of ESRD were insufficient. The goal of this study was to mainly evaluate the prognostic value of eNOS gene polymorphisms and the susceptibility of ESRD.
In the present study, a significant association between ESRD risk and eNOS 4b/a, Glu298Asp polymorphisms was observed in the global population. No association was found between eNOS T708C polymorphism and ESRD risk. According to those results, we conclude that eNOS 4b/a and Glu298Asp would be considered as effective genetic factors, which contributed to the pathology of ESRD.
Nitric oxide (NO), an important intracellular messenger molecule, plays a major role in maintaining homeostasis.Citation35 After being released into endothelial cells, NO diffuses rapidly through cell membranes and relaxes neighboring vascular smooth muscle cells through the production of guanosine 3′,5′-cyclic monophosphate (cGMP).Citation36 A single-nucleotide polymorphism (SNP) in the promoter region (T-786C), a SNP in exon 7 (Glu298Asp) and the variable number of tandem repeats (VNTR) in intron 4 have received most of the attention, because of their association with cardiovascular and renal diseases.
In the 27-bp repeat of intron 4, two alleles have been identified, the larger one, eNOS 4b, has five tandem 27-bp repeats, and the smaller, eNOS 4a, has four repeats.Citation37 Although the precise molecular effects of this polymorphism have not been elucidated, there is biochemical evidence of reduced eNOS expression and enzymatic activity associated with this eNOS 27-bp VNTR.Citation38 Tsukada et al.Citation39 reported that eNOS gene polymorphisms correlate with the circulating NO concentrations, where homozygotes eNOS 4bb and eNOS 4aa exhibit the highest and the lowest NO levels, respectively. Moreover, eNOS 4a allele was associated with the pathogenesis of ESRD. Bellini et al.Citation19 demonstrated a strong correlation between eNOS 4a polymorphism and ESRD risk, while Marson et al.Citation18 found no significant differences in haplotype distribution between eNOS 4a polymorphism and ESRD risk. Our result showed that the frequency of the 4a allele in eNOS intron 4 was significantly higher in patients than in controls (OR = 1.47, 95% CI = 1.05–2.06, p = 0.03), and the frequency of eNOS 4a carrier genotypes (eNOS 4aa and 4ab) was also higher in patients than in healthy controls (p = 0.04), indicating that the eNOS 4a allele may be one of the risk factors for ESRD in ESRD patients.
G894T polymorphism is located in exon 8, which leads to a single amino acid substitution from glutamate to aspartate at the 298 site. The eNOS Glu298Asp variation is fairly widespread in the human population, with 35–40% prevalence of the T allele in Caucasians and less prevalence in African Americans and Asians.Citation40 It is an important gene mutation of eNOS, and Glu298Asp gene polymorphism includes GG (Glu/Glu), GT (Glu/Asp) and TT (Asp/Asp) genotypes, and G (Glu) and T (Asp) alleles. Shear is a key modulator of NOS3 function in vivo and association with caveolae is important for the control of NOS3 protein activity.Citation41 The disease, states most consistently implicating of this SNP, has typically been shown to involve endothelial dysfunction.Citation41 The present epidemiologic study shows that the eNOS Glu298Asp gene polymorphism has been implicated in the etiology of ESRD. However, the available evidence reported to date is weak, due to the sparseness of data or disagreements among studies. In the past years, there were some meta-analyses to explore the association of eNOS Glu298Asp gene polymorphism with the susceptibility of some diseases. Su et al.Citation42 showed significant associations between eNOS Glu298Asp polymorphisms and idiopathic recurrent pregnancy loss. Yu et al.Citation43 found that the eNOS Glu298Asp polymorphism is not associated with a significant increased risk of pre-eclampsia. In our study, we found that T allele was associated with ESRD susceptibility in overall populations (OR = 2.12, 95% CI = 1.44–3.12, p = 0.0001), and GG genotype might play a protective role against ESRD susceptibility in overall populations.
T786C polymorphism is located in the promoter of the eNOS gene at the eNOS-786 site. Molecular exploration indicated that the replacement of thymidine by cytosine is able to cause a reduced promoter activity, which leads to a compromised production of the eNOS enzyme in vivo.Citation44 Our study showed no association between T786C polymorphism and ESRD risk.
In conclusion, an increased susceptibility of eNOS gene 4a (4b/a) and T (G894T) variant carriers to ESRD was observed in the present meta-analysis. GG and 4bb genotype might play a protective role against ESRD susceptibility in overall populations. However, more case-control association studies on larger, stratified populations are required to further elaborate the role of eNOS gene polymorphisms in ESRD susceptibility in different ethnicities.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Sowjanya B, Sreenivasulu U, Naidu JN, Sivaranjani N. End stage renal disease, differential diagnosis, a rare genetic disorder: Bardet-Biedl syndrome: case report and review. Indian J Clin Biochem. 2011;26(2):214–216
- Kuo CC, Lee CT, Ho SC, Kuo HW, Wu TN, Yang CY. Hemodialysis and the risk of stroke: a population-based cohort study in Taiwan, a country of high incidence of end-stage renal disease. Nephrology (Carlton). 2012;17(3):243–248
- Halen NV, Cukor D, Constantiner M, Kimmel PL. Depression and mortality in end-stage renal disease. Curr Psychiatry Rep. 2012;14(1):36–44
- Gilbertson DT, Liu J, Xue JL, et al. Projecting the number of patients with end-stage renal disease in the United States to the year 2015. J Am Soc Nephrol. 2005;16(12):3736–3741
- Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169(4):342–350
- Zhou TB, Yin SS. Association of endothelial nitric oxide synthase Glu298Asp gene polymorphism with the risk of end-stage renal disease. Ren Fail. 2013;35(4):573–578
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327(6122):524–526
- Stuehr DJ. Structure-function aspects in the nitric oxide synthases. Annu Rev Pharmacol Toxicol. 1997;37:339–359
- Marsden PA, Heng HH, Scherer SW, et al. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J Biol Chem. 1993;268(23):17478–17488
- Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–837, 837a–837d
- Nakayama M, Yasue H, Yoshimura M, et al. T-786→C mutation in the 5′-flanking region of the endothelial nitric oxide synthase gene is associated with coronary spasm. Circulation. 1999;99(22):2864–2870
- Lamblin N, Cuilleret FJ, Helbecque N, et al. A common variant of endothelial nitric oxide synthase (Glu298Asp) is associated with collateral development in patients with chronic coronary occlusions. BMC Cardiovasc Disord. 2005;5:27
- Wang XL, Mahaney MC, Sim AS, et al. Genetic contribution of the endothelial constitutive nitric oxide synthase gene to plasma nitric oxide levels. Arterioscler Thromb Vasc Biol. 1997;17(11):3147–3153
- Suzuki H, Nagase S, Kikuchi S, Wang Y, Koyama A. Association of a missense Glu298Asp mutation of the endothelial nitric oxide synthase gene with end stage renal disease. Clin Chem. 2000;46(11):1858–1860
- Walker D, Consugar M, Slezak J, et al. The ENOS polymorphism is not associated with severity of renal disease in polycystic kidney disease 1. Am J Kidney Dis. 2003;41(1):90–94
- Tang FY, Liu FY, Xie XW. Association of angiotensin-converting enzyme and endothelial nitric oxide synthase gene polymorphisms with vascular disease in ESRD patients in a Chinese population. Mol Cell Biochem. 2008;319(1–2):33–39
- Lin S, Qu H, Qu M, Yang X. Association of ecNOS4b/a polymorphism and end-stage chronic renal failure. Chin J Internal Med. 2002;41:513–516
- Marson BP, Dickel S, Ishizawa MH, et al. Endothelial nitric oxide genotypes and haplotypes are not associated with end-stage renal disease. DNA Cell Biol. 2011;30(1):55–59
- Bellini MH, Figueira MN, Piccoli MF, et al. Association of endothelial nitric oxide synthase gene intron 4 polymorphism with end-stage renal disease. Nephrology (Carlton). 2007;12(3):289–293
- Kerkeni M, Letaief A, Achour A, Miled A, Trivin F, Maaroufi K. Endothelial nitric oxide synthetase, methylenetetrahydrofolate reductase polymorphisms, and cardiovascular complications in Tunisian patients with nondiabetic renal disease. Clin Biochem. 2009;42(10–11):958–964
- Tripathi G, Sharma RK, Baburaj VP, Sankhwar SN, Jafar T, Agrawal S. Genetic risk factors for renal failure among north Indian ESRD patients. Clin Biochem. 2008;41(7–8):525–531
- Thaha M, Pranawa, Yogiantoro M, et al. Association of endothelial nitric oxide synthase Glu298Asp polymorphism with end-stage renal disease. Clin Nephrol. 2008;70(2):144–154
- Nagase S, Suzuki H, Wang Y, et al. Association of ecNOS gene polymorphisms with end stage renal diseases. Mol Cell Biochem. 2003;244(1–2):113–118
- Noiri E, Satoh H, Taguchi J, et al. Association of eNOS Glu298Asp polymorphism with end-stage renal disease. Hypertension. 2002;40(4):535–540
- Asakimori Y, Yorioka N, Taniguchi Y, et al. T(-786)→C polymorphism of the endothelial nitric oxide synthase gene influences the progression of renal disease. Nephron. 2002;91(4):747–751
- Asakimori Y, Yorioka N, Yamamoto I, et al. Endothelial nitric oxide synthase intron 4 polymorphism influences the progression of renal disease. Nephron. 2001;89(2):219–223
- Buraczynska M, Ksiazek P, Zaluska W, Nowicka T, Ksiazek A. Endothelial nitric oxide synthase gene intron 4 polymorphism in patients with end-stage renal disease. Nephrol Dial Transplant. 2004;19(9):2302–2306
- Lamnissou K, Zirogiannis P, Trygonis S, et al. Evidence for association of endothelial cell nitric oxide synthase gene polymorphism with earlier progression to end-stage renal disease in a cohort of Hellens from Greece and Cyprus. Genet Test. 2004;8(3):319–324
- El-Din Bessa SS, Hamdy SM. Impact of nitric oxide synthase Glu298Asp polymorphism on the development of end-stage renal disease in type 2 diabetic Egyptian patients. Ren Fail. 2011;33(9):878–884
- de Prado A, Donate T, Martinez E, et al. Endothelial nitric oxide synthase gene polymorphism in dialysis patients. Adv Perit Dial. 2002;18:18–20
- Zsom M, Fulop T, Zsom L, Barath A, Maroti Z, Endreffy E. Genetic polymorphisms and the risk of progressive renal failure in elderly Hungarian patients. Hemodial Int. 2011;15(4):501–508
- Ulrich C, Heine GH, Seibert E, Fliser D, Girndt M. Circulating monocyte subpopulations with high expression of angiotensin-converting enzyme predict mortality in patients with end-stage renal disease. Nephrol Dial Transplant. 2010;25(7):2265–2272
- Sharma R, Agrawal S, Saxena A, Pandey M, Sharma RK. Association of genetic variants of ghrelin, leptin and UCP2 with malnutrition inflammation syndrome and survival in end-stage renal disease patients. Genes Nutr. 2013;8(6):611–621
- Kajimoto H, Kai H, Aoki H, et al. Inhibition of eNOS phosphorylation mediates endothelial dysfunction in renal failure: new effect of asymmetric dimethylarginine. Kidney Int. 2012;81(8):762–768
- Jensen FB. The role of nitrite in nitric oxide homeostasis: a comparative perspective. Biochim Biophys Acta. 2009;1787(7):841–848
- Figueroa XF, Lillo MA, Gaete PS, Riquelme MA, Saez JC. Diffusion of nitric oxide across cell membranes of the vascular wall requires specific connexin-based channels. Neuropharmacology. 2013;75:471–478
- Umar S, van der Laarse A. Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic, and failing heart. Mol Cell Biochem. 2010;333(1–2):191–201
- Song J, Yoon Y, Park KU, et al. Genotype-specific influence on nitric oxide synthase gene expression, protein concentrations, and enzyme activity in cultured human endothelial cells. Clin Chem. 2003;49(6 Pt 1):847–852
- Tsukada T, Yokoyama K, Arai T, et al. Evidence of association of the ecNOS gene polymorphism with plasma NO metabolite levels in humans. Biochem Biophys Res Commun. 1998;245(1):190–193
- Andersen ML, Guindalini C, Santos-Silva R, Bittencourt LR, Tufik S. Association analysis of endothelial nitric oxide synthase G894T gene polymorphism and erectile dysfunction complaints in a population-based survey. J Sex Med. 2010;7(3):1229–1236
- Joshi MS, Mineo C, Shaul PW, Bauer JA. Biochemical consequences of the NOS3 Glu298Asp variation in human endothelium: altered caveolar localization and impaired response to shear. FASEB J. 2007;21(11):2655–2663
- Su MT, Lin SH, Chen YC. Genetic association studies of angiogenesis- and vasoconstriction-related genes in women with recurrent pregnancy loss: a systematic review and meta-analysis. Hum Reprod Update. 2011;17(6):803–812
- Yu CK, Casas JP, Savvidou MD, Sahemey MK, Nicolaides KH, Hingorani AD. Endothelial nitric oxide synthase gene polymorphism (Glu298Asp) and development of pre-eclampsia: a case-control study and a meta-analysis. BMC Pregnancy Childbirth. 2006;6:7
- Nakayama M, Yasue H, Yoshimura M, et al. T(-786)→ C mutation in the 5′-flanking region of the endothelial nitric oxide synthase gene is associated with myocardial infarction, especially without coronary organic stenosis. Am J Cardiol. 2000;86(6):628–634