Abstract
We aimed to evaluate the cancer detection rates of 6-, 10-, 12-core biopsy regimens and the optimal biopsy protocol for prostate cancer diagnosis in patients with renal failure. A total of 122 consecutive patients with renal failure underwent biopsy with age-specific prostate-specific antigen (PSA) levels up to 20 ng/mL. The 12-core biopsy technique (sextant biopsy + lateral base, lateral mid-zone, lateral apex, bilaterally) performed to all patients. Pathology results were examined separately for each sextant, 10-core that exclude parasagittal mid-zones from 12-cores (10a), 10-core that exclude apex zones from 12-cores (10b) and 12-core biopsy regimens. Of 122 patients, 37 (30.3%) were positive for prostate cancer. The cancer detection rates for sextant, 10a, 10b and 12 cores were 17.2%, 29%, 23.7% and 30.7%, respectively. Biopsy techniques of 10a, 10b and 12 cores increased the cancer detection rates by 40%, 27.5% and 43.2% among the sextant technique, respectively. Biopsy techniques of 10a and 12 cores increased the cancer detection rates by 17.1% and 21.6% among 10b biopsy technique, respectively. There were no statistical differences between 12 core and 10a core about cancer detection rate. Adding lateral cores to sextant biopsy improves the cancer detection rates. In our study, 12-core biopsy technique increases the cancer detection rate by 5.4% among 10a core but that was not statistically different. On the other hand, 12-core biopsy technique includes all biopsy regimens. We therefore suggest 12-core biopsy or minimum 10-core strategy incorporating six peripheral biopsies with elevated age- specific PSA levels up to 20 ng/mL in patients with renal failure.
Introduction
The wide spread use of prostate-specific antigen (PSA) and transrectal ultrasound (TRUS)-guided needle biopsy dramatically improved the detection rate of early prostate cancer. Since Hodge et al. first introduced TRUS-guided sextant prostate biopsy, it has been the most widely used method for diagnosing prostate cancer.Citation1 Sextant biopsy false negative cancer detection rates ranged from 20 to 35% according to the clinical characteristics of studied population.Citation2,Citation3 After discovery of prostate cancer stems from the most common prostate peripheral zone, the importance of the need for a very good sampling of the peripheral zone started to be questioned during the diagnosis. Eskew et al. reported that cancer detection rate would increase up to 35% with 13-core biopsy strategy.Citation4 In 1999, Chen et al. reported that, the highest cancer detection rate was achieved with the 11-core biopsy strategy, based on computer simulations of radical prostatectomy specimens.Citation4,Citation5 Ellabady et al. reported extended 12-core prostate biopsy significantly increases both the detection rate of prostate cancer and the accuracy of biopsy Gleason score.Citation6
There have been improvements in survival rates of patients with renal failure.Citation7 As the survival time of patients with renal failure has increased, the number of elderly patients with renal failure has also increased.Citation7 Thus, the potential for development of prostate cancer has become a real concern, given the increase in elderly patients with renal failure.Citation8 This study was conducted to evaluate the diagnostic yield of TRUS-guided 6-, 10-, 12-core prostate biopsy regimens in patients with renal failure.
Materials and methods
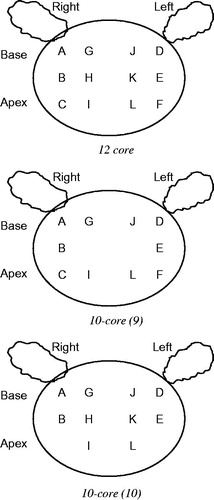
Between December 2004 and October 2013, 122 consecutive men with renal failure and suspected prostate cancer were included in the study. Renal failure or chronic kidney disease (CKD) was defined as glomerular filtration rate (GFR) less than 60 mL/min per 1.73 m2 according to CKD stage 3 (moderate decrease of GFR: 30–59 mL/min per 1.73 m2).Citation9 Indications for TRUS-guided prostate biopsy were: abnormal digital rectal examination and/or a serum PSA over 2.5 ng/mL and less than 20 ng/mL. None of these patients had previous prostate biopsies. All patients were thoroughly examined by TRUS before biopsy and prostate volumes were calculated using an ellipsoid prostate formula. All of the lesions detected by TRUS were noted. Local anesthesia of 5 cc lidocaine (1%) was applied to prostate apex and base. All of the patients were placed in the left lateral decubitus position and all were examined with no bowel preparation. We used an 18 G core biopsy needle mounted on a spring-loaded automatic biopsy gun. The 12-core biopsy technique (sextant biopsy + lateral base, lateral mid-zone, lateral apex, bilaterally) performed to all patients. Besides the sextant technique, six more biopsies were obtained from the lateral peripheral zones of base, mid-zone and apex of the prostate, as depicted in . Pathology results were examined separately for each sextant described by Hodge et al.,Citation1 10 core that exclude parasagittal mid zones from 12 cores (10a) described by Gore et al. (),Citation10 10 core that exclude apex zones from 12 cores (10b) described by Presti et al. ()Citation11 and 12-core biopsy regimens. All patients underwent the same biopsy protocol regardless of the prostate gland size. Pathology results were examined separately for each sextant, 10 core that exclude parasagittal mid zones from 12 cores (10a), 10 core that exclude apex zones from 12 cores (10b) and 12-core biopsy regimens. All of the patients tolerated the biopsy procedure well and none of the patients needed intravenous sedation or narcotic analgesics. Informed consent was obtained from each patient. The Institutional Review Board approved the clinical protocol.
Figure 1. Location of prostate biopsy schemes. A: Right base lateral, B: Right middle lateral, C: Right apex lateral, D: Left base lateral, E: Left middle lateral, F: Left Apex lateral, G: Right base middle, H: Right middle middle, I: Right apex middle, J: Left base middle, K: Left middle middle, L: Left apex middle.
SPSS for Windows 11.0’ (Chicago, IL) was used for statistical analysis of the data. Chi-square test was used for statistical analysis and p < 0.05 was considered statistically significant.
Results
Median patients age was 62 (range 42–93). Median pre-biopsy PSA was 8.4 ng/mL (range 2.4–20) and median prostate volume was 51 cc (range 13–139). The mean disease duration of renal failure was 3.58 years. Thirty-seven (30.3%) of 122 patients had prostate adenocarcinoma at biopsy. The mean age, mean PSA value and mean prostate volume of the patients with prostate cancer was 66.7 years, 10.2 ng/mL, 43.48 cc, respectively. On the other hand, the mean age, mean PSA value and mean prostate volume of the patients with no prostate cancer at biopsy was 63 years, 7.7 ng/mL, 54.46 cc, respectively. The overall patients’ age and PSA value were significantly higher in prostate adenocarcinoma group. The overall prostate volume was significantly higher in benign prostatic hyperplasia (BPH) group ().
Table 1. Demographic data of patients.
Of the 37 cancers diagnosed, total 137 cores were positive and of these positive cores 61 (44%) was on the standard sextant biopsy location and 76 (56%) was on lateral biopsy location (p < 0.005). On the focal analysis the most common cancer localizations were bilateral apex lateral cores (total 23.07%). Pathological reports of all 122 patients were analyzed separately each for sextant, 10a, 10b and 12 cores and cancer detection rates of each protocol was 17.2% (21/122), 29% (35/122), 23.7% (29/122) and 30.7% (37/122), respectively ().
Table 2. Biopsy technique and cancer detection rates.
Sextant biopsy technique compared with 10a-, 10b- and 12-core techniques and we found 10a-, 10b- and 12-core biopsy techniques increased cancer detection rates according to sextant biopsy 40% (14/35), 27.5% (8/29) and 43.2% (16/37), respectively (p < 0.005). When we compare 10b biopsy technique with 10a- and 12-core biopsy techniques, we found 10a- and 12-core biopsy techniques increased cancer detection rates according to 10b biopsy technique 17.1% (6/35) and 21.6% (8/37), respectively (p < 0.005). Only 2 (5.4%) patients dismissed with 10a biopsy technique according to 12-core biopsy techniques and when we compared 10a biopsy technique and 12-core biopsy techniques there were no statistically differences between cancer detection rates (p > 0.005; ).
Table 3. Comparison of biopsy techniques.
Discussion
Studies previously have reported a higher incidence of malignant diseases in patients with renal failure than in the general population, and this tendency is marked among patients with prostate cancer.Citation12–15 This higher incidence could be explained by several reasons as follows: presence of chronic infection particularly in the urinary tract, weakened immune system, nutritional deficiencies and altered DNA repair.Citation16 Furthermore, the age of patients with renal failure has increased with progression in the field of therapy, suggesting a proportional increase in the prevalence of prostate cancer among these patients.Citation17 Recently, it is also reported that in patients with renal failure age-stratified PSA was higher than in the general population. And the cancer detection rate was increased in patients with renal failure compared to that in patients with normal renal function at specific PSA intervals.Citation15 The authors concluded that lower PSA cutoffs may be appropriate to recommend prostate biopsy in patients with end-stage renal disease.Citation15
Another study investigating the association of CKD and cancer risk in older people reported that the risk for lung and urinary tract cancers but not prostate was higher among men with CKD.Citation18 The results of that study show no site-specific increased risk for prostate cancer in men. Prostate cancer incidence rate was 4.71% in patients with a GFR of <60 mL/min per 1.73 m2 and 4.75% in patients with a GFR of ≥60 mL/min per 1.73 m2. Reduced kidney function to the lowest threshold of GFR (<40 mL/min) was significantly associated with lung and urinary tract cancers in men but showed no significant association for colorectal, breast, and prostate cancers after adjustment for the effect of age, smoking status, sun-related skin damage, and diastolic blood pressure.Citation18
TRUS-guided systematic prostate biopsy remains the gold standard test for diagnosing prostate cancer. Since Hodge et al. first introduced (TRUS)-guided sextant prostate biopsy, it has been the most widely used method for diagnosing prostate cancer but numerous studies have demonstrated that increasing the number of cores sampled during prostatic biopsy will increase the detection rate for prostate cancer. Authors suggest that sextant biopsy regimen is not sufficient about number and localization of cores and they report 20–35% of false-negative results.Citation6,Citation19,Citation20 In a systematic review, Eichler et al. analyzed 87 studies with a total of 20,698 patients and they pooled data from 68 studies. They reported that increasing number of biopsy cores were significantly associated with the cancer yield. Schemes with 12 cores that took additional laterally directed cores detected 31% more cancers than the sextant scheme and taking more than 12 cores added no significant benefit.Citation21 In our study, false-negative result for sextant biopsy was 44% and 16 patients have to be dismissed if only the sextant biopsy was performed. In 1995, Stamey suggested shifting the sextant biopsies more laterally in order to sample better the peripheral zone where most of the cancers are located.Citation22 Chen et al. compared the ability of different biopsy schemes to detect cancer and predict tumor volume using prostate biopsy simulation system in 180 radical prostatectomy specimens. A total of 1,180,800 individual biopsy tracks were simulated and they suggest that the detection rate of prostate biopsies is not related solely to the number of cores taken, core placement is also important.Citation5 In our study 56% of prostate cancer located on laterally biopsy cores of 76 cancer focus. We also observed the most common diagnosed cancer foci was bilaterally apex laterals (total 23%). Presti et al. added lateral biopsies of the peripheral zone at the base and mid-gland to the routine sextant biopsy regimen for a total of 10 systematic biopsies and they found traditional sextant biopsies missed 20%.Citation11 In our study, we found that this procedure increased the cancer detection rate 27.5% among sextant biopsy but missed 21.6% cancer via 12-core biopsy protocol. Gore et al. in 2001 compared the sensitivity of different combinations of biopsy cores with those of standard sextant biopsies and with a 12-core biopsy protocol that combined the standard sextant biopsy with a complete set of laterally directed cores. They found combinations of biopsy cores a strategy that included laterally directed cores at the base, mid-gland and apex of the prostate with mid lobar and apical base cores detected 98.5% of cancers and detection rate of this 10-core biopsy regimen was significantly better than that of the standard sextant protocol, and was equivalent to that of the 12-core regional biopsy.Citation10 In our study, Gore protocol (10a) was increased the cancer detection rate among sextant biopsy and Presti (10b) model were 40% and 17.1%, respectively, and cancer detection rate was 94.6% compared with 12-core biopsy. Gore technique includes bilaterally lateral apex cores and these regions were the most cancer detected focus in our study.
The purpose of prostate biopsies was to detect prostate cancer with minimum inconvenience of the patient whether with optimal biopsy cores and localizations. In our study, 12-core biopsy protocol in patients with renal failure increases the cancer detection rate 5.4% among Gore (10a) biopsy protocol but that was not statistically significant. On the other hand, 12-core biopsy technique include other biopsy techniques and provide the all lateral cores sampling; thus, we suggest 12-core biopsy or minimum 10-core strategy incorporating at least six peripheral biopsies with age-specific PSA value 20 ng/mL in patients with renal failure.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hodge KK, Mcneal JE, Terris MK, Stamey TA. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol. 1989;142:71–75
- Stricker HJ, Ruddock LJ, Wan J, Belville WD. Detection of nonpalpable prostate cancer. A mathematical and laboratory model. Br J Urol. 1993;71:43–46
- Eskiçorapçı SY, Baydar DE, Akbal C, et al. An extended 10 core transrectal ultrsonography guided prostate biopsy protocol improves the detection of prostate cancer. Eur Urol. 2004;45:444–449
- Eskew LA, Bare RL, McCullough DL. Systematic 5 region prostate biopsy is superior to sextant method diagnosing carcinoma of the prostate. J Urol. 1997;157:199–203
- Chen ME, Troncoso P, Tang K, Babaian RJ, Johnston D. Comparison of prostate biopsy schemes by computer simulation. Urology. 1999;53:951–960
- Elabbaday AA, Khedr MM. Extended 12 core prostate biopsy increase both te detection of prostate cancer and the accuracy of Gleason score. Eur Urol. 2006;49:49–53
- Nakai S, Shinzato T, Nagura Y, et al. Patient Registration Committee, Japanese Society for Dialysis Therapy, Tokyo. An overview of regular dialysis treatment in Japan (as of 31 December 2001). Ther Apher Dial. 2004;8(1):3–32
- Wada Y, Nakanishi J, Takahashi W, et al. Mass screening for prostate cancer in patients with end-stage renal disease: a comparative study. BJU Int. 2006;98:794–797
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266
- Gore JL, Shariat SF, Miles BJ, et al. Optimal Combination of systematic sextant and laterally directed biopsies for the detection of prostate cancer. J Urol. 2001;165:1554–1559
- Presti JC, Chang JJ, Bhargava V, Shinohara K. The optimal systematic prostate biopsy scheme should include 8 rather than 6 biopsies: results of a prospective clinical trial. J Urol. 2000;163:163–166
- Maisonneuve P, Agodoa L, Gellert R, et al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet. 1999;354:93–99
- Palestini M, Lucandri G, Sterpetti A, Izzo L, Orefici F, Cavallaro A. Cancer surveillance in patients receiving long-term hemodialysis. Anticancer Res. 2002;22:1305–1310
- Cengiz K. Increased incidence of neoplasia in chronic renal failure (20-year experience). Int Urol Nephrol. 2002;33:121–126
- Chen CJ, Heldt JP, Anderson KM, et al. Prostate specific antigen levels and prostate cancer detection rates in patients with end stage renal disease. J Urol. 2012;187(6):2061–2065
- Vamvakas S, Bahner U, Heidland A. Cancer in end stage renal disease: potential factors involved. Am J Nephrol. 1998;18:89–95
- Kurahashi T, Miyake H, Shinozaki M, et al. Screening for prostate cancer using prostate-specific antigen testing in Japanese men on hemodialysis. Int Urol Nephrol. 2008;40:345–349
- Wong G, Hayen A, Chapman JR, et al. Association of CKD and cancer risk in older people. J Am Soc Nephrol. 2009;20:1341–1350
- Bauer JJ, Zeng J, Weir J, et al. Three-dimensional computer-simulated prostate models: lateral prostate biopsy cores increase the detection rate of prostate cancer. Urology. 1999;53:961–967
- Babaian RJ, Toi A, Kamoi K, et al. A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. J Urol. 2000;163:152–157
- Eichler K, Hempel S, Wilby J, Myers L, Bachmann LM, Kleijnen J. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol. 2006;175:1605–1612
- Stamey TA. Making the most out of six systematic sextant biopsy. Urology. 1995;45:71–74
