Abstract
IgA nephropathy (IgAN) has become the most common form of primary glomerular disease worldwide. So far, it is still not very clear about the exact pathogenesis of IgAN, thus has no specific therapy. Generally mesangial deposition of IgA, especially polymeric IgA1 (pIgA1), suggests to be the initiating event in the pathogenesis of IgAN. In addition to decreased IgA clearance, IgA over production may also participate in the pathogenesis of IgAN. IgA class switching recombination (CSR) played key role during the process of IgA production. Stimulated with hemolytic streptococcus, tonsillar mononuclear cells (TMCs) of patients with IgAN presented with increased levels of Ia-Ca and activation-induced cytidine deaminase (AID), which are significant for IgA CSR. Human B cells and plasmacytoid dendritic cells express Toll-like receptor (TLR)-9, whose natural ligands are unmethylated cytosine–guanine dinucleotide (CpG) motifs characteristic of bacterial DNA (CpG-DNA). Unmethylated deoxycytidylic-deoxyguanosine oligodeoxynucleotide (CpG-ODN) is able to mimic the immunostimulatory activity of microbial DNA. Study found a significant increase in B cell activation factor (BAFF) production when tonsillar mononuclear cells stimulated with CpG-ODN in patients with IgAN. BAFF can induce germline Cα gene expression, AID expression, and IgA class switching in a CD40-independent manner. Therefore, it could be hypothesized that in IgAN there may exist TLR9-BAFF-IgA CSR axis, which induces excessive IgA production. If the hypothesis is correct, it could be of great significance for pathogenesis of IgAN elucidate and IgAN treatment.
Introduction
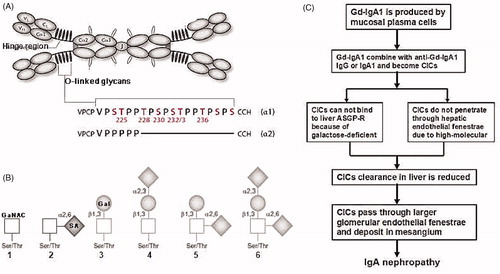
As the most common primary glomerular disease worldwide, IgA nephropathy (IgAN) is characterized by an overrepresentation of IgA1 molecules in the serum and mesangial deposition of IgA immune complexes.Citation1 In human, there are two subclasses IgA, IgA1 and IgA2. Compared with IgA2, the major difference of IgA1 is the presence of hinge region, which is rich in serine, praline, and threonine, and having a variable number of O-linked oligosaccharides (galactose, Gal or N-acetylgalactosamine, GalNAc) (). In healthy person, IgA1 in circulation could be combined with hepatic asialoglycoprotein receptor (ASGP-R) through a terminal Gal or GalNAc and is catabolized in the liver. As compared with healthy persons, IgAN patients have more circulating IgA1 molecules with O-linked hinge-region glycans that do not include galactose (), which is easy to combine with circulating antibodies into circulating immune complex (CIC). Because of higher molecule, these CICs do not pass through the hepatic endothelial fenestrae to enter the space of Disse, so they avoid contact with the hepatocyte-expressed ASGP-R, therefore, reduce IgA1 catabolism. Finally, CICs enter the glomerular capillaries, where they pass through larger endothelial fenestrae to deposit in the mesangium and result in IgAN (). However, in addition to the traditional view that decreased hepatic removal of IgA induce IgANCitation2,Citation3; a lot of evidences indicate IgA excessive-generation take part in the pathogenesis of IgAN.
Figure 1. General viewpoint about IgAN pathogenesis.2,3 (A) Structure of human polymeric IgA1 (pIgA1) and the O-glycosylation sites. pIgA1 occurs predominantly in a dimeric form that is composed of two IgA1 monomers and J chain. In IgA between the first (Cα1) and second (Cα2) constant-region domains is hinge region (HR) which is different in IgA1 (chain α1) and IgA2 (chain α2). Within IgA1, HR is rich in proline (Pro/P), threonine (Thr/T), and serine (Ser/S) amino acid residues and up to six glycans are attached to an oxygen molecule of a serine or threonine residue (O-linked). (B) O-glycan variants of IgA1. In healthy person, Thr and Ser residues in IgA1 HR are normally extended like the nos. 3–6, while in IgAN patients are as the nos. 1 and 2. (C) Sketch of IgA1 metabolism in IgAN. GaNAC, N-acetylgalactosamine; Gal, galactose; SA, sialic acid; Gd-IgA1, galactose-deficient IgA1; CICs, circulating immune complex; ASGP-R, asialoglycoprotein receptor.
Clinically, urinary findings deterioration frequently emerged after upper respiratory infections, especially tonsillar focal infection. Great evidences revealed that as one of the mucosa-associated lymphoid tissues (MALT) tonsils were closely related to IgANCitation4 and tonsillectomy can improve the urinary findings, keep stable renal function, improve mesangial proliferation, decrease IgA deposits, and have a favorable effect on long-term renal survival in some IgAN patients.Citation5,Citation6 Previously, we have isolated and identified the tonsil bacterial expression profile of IgAN patients and non-nephritis chronic tonsillitis patients. α-Hemolytic streptococci were detected in all IgAN patients.Citation7 Now, it has been confirmed that the hemolytic streptococcus contains unmethylated cytosine–guanine dinucleotide (CpG) motifs characteristic of bacterial DNA (CPG-DNA), which can activate Toll-like receptor (TLR)-9 of human B cells and plasmacytoid dendritic cells (pDC). As one new member of tumor necrosis factor (TNF) family, B-cell-activation factor (BAFF) plays an important role in IgA class switching recombination (CSR), which is significant for IgA production. In patients with IgAN, serum BAFF is increased and associated with clinical and histopathological features.Citation8 Thus, it suggests that TLR9, BAFF, and IgA CSR are closely related with IgA hyper-production and result in IgAN. Here, we discuss the role of TLR9, BAFF, and IgA CSR in pathogenesis of IgAN and relationships between them, providing evidence to disclose the pathogenesis of IgAN and assistance for disease treatment.
TLR9: what is the role of it in IgAN after upper respiratory infections?
TLR are a family of pathogen pattern recognition receptors that recognize different types of pathogen-related structures and activate defense mechanisms, especially in innate immunity.Citation9,Citation10 TLR9 has been identified as a natural receptor for bacterial and viral DNA containing a specific sequence pattern including CpG-DNA.Citation11 Oligodeoxynucleotides (ODNs) with CpG (CpG-ODN) are able to perfectly mimic the immunostimulatory activity of microbial DNA.Citation12 Kajiyama et al.Citation13 nasally challenged cell-specific CpG-ODN and found that CpG-ODN administration aggravated urinary albumin and glomerular injury in IgAN prone mice, in comparison with the aggravation in the ddY vehicle control group, while CpG-ODN administration caused no elevation in urinary albumin excretion and/or glomerular damage in normal Bal b/c mice. Their report is consistent with clinical findings that urinary deterioration emerged after upper respiratory infections. MALT stimulated with CpG-DNA can result in hyper-production of IgA and TLR9 plays an important role during this process. In patients with Kawasaki disease, activation of B lymphocytes stimulated by CpG-ODN induced a significant increase in the number of IgA-secreting cells in acute-phase compared with age-matched controls.Citation14 Our previous study revealed that IgA and IgA1 levels in tonsillar mononuclear cells (TMCs) stimulated with 10 μg/mL of lipopolysaccharide or 1 × 108 cfu/mL of hemolytic streptococcus were markedly increased compared with the control group in patients with IgAN.Citation15 High expression of tonsillar TLR9 was observed in some IgAN patients, who showed stronger and earlier remission of hematuria and proteinuria than those with low TLR9 expression after tonsillectomy with steroid pulse therapy (SPT).Citation16 Taken together, MALT infections induce CpG-DNA in pathogenic bacterium recognized by TLR9 and up-regulate IgA production, finally result in aggravation of IgAN.
BAFF: the medium of TLR9 and IgA hyper-production in IgAN
However, what is the mechanism of TLR9 to induce elevated IgA production in IgAN? Goto et al.Citation17 reported that the BAFF production of TMCs stimulated with CpG-ODN was significantly higher in IgAN patients than that in non-IgAN patients, while without any stimulation the BAFF production was not different between IgAN patients and non-IgAN patients. Next they investigated how CpG-ODN stimulation influence cytokine productions and suggested that IFN-γ induced by CpG-ODN stimulation may contribute to IgA production via up-regulation of BAFF production, resulting in hyperproduction of IgA via activation of B-1 cells in tonsils of IgAN patients. As a new member of tumor necrosis factor (TNF) family, BAFF (also known as TALL-1, THANK, BlyS, and zTNF4) plays a key role in B-cell development and IgA production.Citation18,Citation19 There are three distinct BAFF receptors that are B cell-activating factor of the TNF family receptor (BAFF-R), transmembrane activator, and calcium-modulator and cyclophilin ligand interactor (TACI) and B cell maturation antigen (BCMA).Citation20 With respect to IgA production, BAFF has been shown to induce T-cell-independent class switching to IgA.Citation21 In BAFF-overexpressing transgenic (BAFF-Tg) mice, the level of serum IgA and the frequency of IgA+ plasma cells in intestinal lamina propria (LP) were significantly elevated compared with age-matched controls. And these observations significantly correlate with IgA deposition in the glomerular mesangium.Citation22 In human, serum BAFF is elevated in patients with IgAN than controls and associated with clinical and histopathological features, including the levels of eGFR, serum creatinine, 24-h proteinuria, serum blood urea nitrogen, and serum uric acid, and the scores for mesangial hypercellularity, segmental glomerulosclerosis, and tubular atrophy/interstitial fibrosis.Citation8 Furthermore, a study reported that in BAFF-Tg mice only with the participation of bacterial signals or antigens can induce increased IgA production and IgA deposition in the kidney.Citation23 Therefore, we may conclude that after MALT particularly tonsils infected by pathogenic bacterium which containing CpG-DNA, TLR9 recognize CpG-DNA and induce production of cytokines, for IFN-γ instance, which up-regulate BAFF production, finally lead hyper-production of IgA.
IgA CSR: in response to BAFF it induced IgA hyper-production in IgAN
From the above data, we know that TLR9-BAFF signaling axis can induce hyper-production of IgA in IgAN. But what is the specific mechanism of BAFF induce IgA production after MALT infections in IgAN? It is generally suggested that BAFF regulated IgA production through affecting B cell proliferation and function.Citation18 But in our previous study, we stimulated TMCs of patients with IgAN by hemolytic streptococcus and found that not only levels of IgA and IgA1 significantly increased but also elevated expression levels of Ia-Ca and activation-induced cytidine deaminase (AID).Citation15 As members of the apolipoprotein B mRNA-enzyme catalytic polypeptide (APOBEC) cytidine deaminase family of proteins, AID is expressed in B cells inducing class switch recombination of the μ constant region to γ, α, and ξ, thereby changing the antibody isotype from IgM to IgG, IgA, and IgE.Citation7,Citation24 In addition, with other cytokines, assistance BAFF can induce germline Cα gene expression, AID expression, and IgA class switching in a CD40-independent manner.Citation21,Citation25 It may be speculated that in IgAN BAFF induce increased IgA production through regulating IgA CSR.
Antibody diversification is essential for the immune system to mount protective humoral responses. In the presence of antigen, class switching is one of the important forms that mature B cells diversify their antibody repertoire.Citation26 IgA class switching is the process whereby B cells acquire the expression of IgA. It occurs in the constant region of the immunoglobulin heavy chain (CH), from Cu to Ca, and is accomplished by T-cell-dependent and T-cell-independent CSR.Citation27 T-cell-independent CSR is induced by two dendritic cell-derived cytokines, a proliferation-inducing ligand (APRIL) and BAFF.Citation21,Citation25 Expression of the TACI receptor by B cells plays significant role in IgA class switching induced by APRIL and BAFF, as B cells lacking TACI do not express AID or undergo CSR in response to BAFF or APRIL.Citation25 Furthermore, TACI deficient mice exhibit decreased steady-state serum IgA levels and make less IgA in response to T-cell-independent antigens.Citation28 In summary, it suggests that IgA class switching is highly dependent on the engagement of TACI on B cells by BAFF and APRIL. TACI is supposed to induce tumor necrosis factor receptor -associated factor (TRAF)-dependent activation of the inhibitor of KappaB kinase (IKK) complex, followed by nuclear translocation of nuclear factor (NF)-κB.Citation29 This signaling axis may be crucial to induce AID expression.Citation30,Citation31
Consequences of the hypothesis and summary
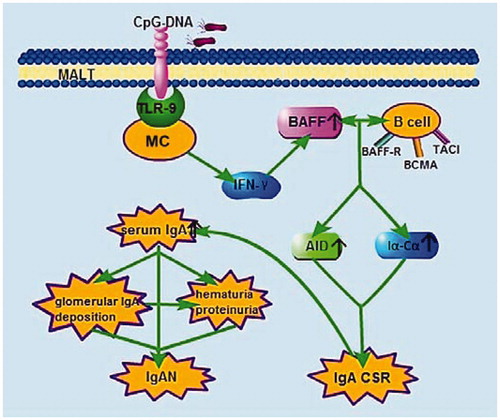
According to the literature reviewed above, we speculate that there may be TLR9-BAFF-IgA CSR signaling axis in IgAN to induce IgA over production (), although further investigations need to do. After tonsillar focal infection, CPG-DNA in pathogenic bacteria recognized by TLR9 and induced cytokines releasing, the cytokines up-regulate expression of BAFF, which result in increased IgA production through IgA CSR. If the hypothesis is to be confirmed, it would be helpful for the pathogenesis of IgAN to elucidate, furthermore, altering or blocking this signaling axis might be useful in the prevention and treatment of IgAN.
Figure 2. Sketch of TLR9-BAFF-IgA CSR signaling axis result in IgA over production. TLR9 can be expressed by mononuclear cells (MC), such as B cells and plasmacytoid dendritic cells, and its natural ligand is CpG-DNA. After MALT (for example tonsil) was infected, CpG-DNA in pathogenic bacteria recognized by TLR9 and induced cytokines releasing, mainly IFN-γ. These cytokines up-regulate expression of BAFF, which interact with B cells and induce CD40-independent IgA CSR. Finally, IgA, especially IgA1, was over produced, therefore, aggravate and lead to IgAN. MALT, mucosa-associated lymphoid tissues.
Declaration of interest
The authors have no conflicts of interest to disclose. This article was supported by grants from the Key Program (81170663) of the National Natural Science Foundation of China and Program (12JJ6094) of the Natural Science Foundation of Hunan province.
References
- Julian BA, Novak J. IgA nephropathy: an update. Curr Opin Nephrol Hypertens. 2004;13(2):171–179
- Robert JW, Bruce AJ. IgA nephropathy. N Engl J Med. 2013;368:2402–2414
- Mestecky J, Raska M, Julian BA, et al. IgA nephropathy: molecular mechanisms of the disease. Annu Rev Pathol. 2013;8:217–240
- Itoh A, Iwase H, Takatani T, et al. Tonsillar IgA1 as a possible source of hypoglycosylated IgA1 in the serum of IgA nephropathy patients. Nephrol Dial Transplant. 2003;18(6):1108–1114
- Ochi A, Moriyama T, Takei T, Uchida K, Nitta K. Comparison between steroid pulse therapy alone and in combination with tonsillectomy for IgA nephropathy. Int Urol Nephrol. 2013;45(2):469–476
- Maeda I, Hayashi T, Sato KK, et al. Tonsillectomy has beneficial effects on remission and progression of IgA nephropathy independent of steroid therapy. Nephrol Dial Transplant. 2012;27(7):2806–2813
- Huang H, Peng Y, Liu H, et al. Effect of tonsillar inactivate strain on tonsillar CD4+CD25+ cells and J chain production in patients with IgA nephropathy[J]. Nephrol Dial Transplant (Chinese). 2007;3(16):215–221
- Xin G, Shi W, Xu LX, Su Y, Yan LJ, Li KS. Serum BAFF is elevated in patients with IgA nephropathy and associated with clinical and histopathological features. J Nephrol. 2013;26(4):683–690
- Janeway CA Jr, Medzhitov R. Medzhitov. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–680
- Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374(6522):546–549
- Kajiyama T, Suzuki Y, Kihara M, Suzuki H, Horikoshi S, Tomino Y. Different pathological roles of toll-like receptor 9 on mucosal B cells and dendritic cells in murine IgA nephropathy. Clin Dev Immunol. 2011;2011:819646 . doi: 10.1155/2011/819646
- Giordani L, Quaranta MG, Marchesi A, et al. Increased frequency of immunoglobulin (Ig)A-secreting cells following Toll-like receptor (TLR)-9 engagement in patients with Kawasaki disease. Clin Exp Immunol. 2011;163(3):346–353
- Liu H, Peng Y, Liu F, Xiao W, Zhang Y, Li W. Expression of IgA class switching gene in tonsillar mononuclear cells in patients with IgA nephropathy. Inflamm Res. 2011;60(9):869–878
- Sato D, Suzuki Y, Kano T, et al. Tonsillar TLR9 expression and efficacy of tonsillectomy with steroid pulse therapy in IgA nephropathy patients. Nephrol Dial Transplant. 2012;27(3):1090–1097
- Goto T, Bandoh N, Yoshizaki T, et al. Increase in B-cell-activation factor (BAFF) and IFN-gamma productions by tonsillar mononuclear cells stimulated with deoxycytidyl-deoxyguanosine oligodeoxynucleotides (CpG-ODN) in patients with IgA nephropathy. Clin Immunol. 2008;126(3):260–269
- Schneider P, MacKay F, Steiner V, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189(11):1747–1756
- Batten M, Fletcher C, Ng LG, et al. TNF deficiency fails to protect BAFF transgenic mice against autoimmunity and reveals a predisposition to B cell lymphoma. J Immunol. 2004;172(2):812–822
- Thompson JS, Bixler SA, Qian F, et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293(5537):2108–2111
- Litinskiy MB, Nardelli B, Hilbert DM, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3(9):822–829
- McCarthy DD, Chiu S, Gao Y, Summers-deLuca LE, Gommerman JL. BAFF induces a hyper-IgA syndrome in the intestinal lamina propria concomitant with IgA deposition in the kidney independent of LIGHT. Cell Immunol. 2006;241(2):85–94
- McCarthy DD, Kujawa J, Wilson C, et al. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest. 2011;121(10):3991–4002
- Harris RS, Sheehy AM, Craig HM, Malim MH, Neuberger MS. DNA deamination: not just a trigger for antibody diversification but also a mechanism for defense against retroviruses. Nat Immunol. 2003;4(7):641–643
- Castigli E, Wilson SA, Scott S, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201(1):35–39
- Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–292
- Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol. 2003;3(1):63–72
- von Bülow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14(5):573–582
- Xia XZ, Treanor J, Senaldi G, et al. TACI is a TRAF-interacting receptor for TALL-1, a tumor necrosis factor family member involved in B cell regulation. J Exp Med. 2000;192(1):137–143
- Mackay F, Schneider P. TACI, an enigmatic BAFF/APRIL receptor, with new unappreciated biochemical and biological properties. Cytokine Growth Factor Rev. 2008;19(3–4):263–276
- Dedeoglu F, Horwitz B, Chaudhuri J, Alt FW, Geha RS. Induction of activation-induced cytidine deaminase gene expression by IL-4 and CD40 ligation is dependent on STAT6 and NFkappaB. Int Immunol. 2004;16(3):395–404
