Abstract
Cisplatin is one of the commonly used anticancer drugs and nephrotoxicity limits its use. The aim of this study is to investigate the possible protective effect of creatine supplementation on cisplatin-induced nephrotoxicity. Sixty male Sprague–Dawley rats were divided into three groups: Group I: Cisplatin (n = 20) (7 mg/kg cisplatin intraperitoneal (i.p.) single dose), group II: Cisplatin + creatine monohydrate (n = 20) (7 mg/kg cisplatin i.p. single dose and 300 mg/kg creatine p.o. daily for 30 days starting on first day of cisplatin injection), group III: Control group (n = 20) (Serum physiologic, 2.5 mL/kg i.p.). Sacrifications were performed at first week and 30th day. Blood urea nitrogen (BUN) and serum creatinine levels, histopathological evaluation, mitochondrial deoxyribonucleic acid (mtDNA) common deletion rates, and body weights of rats were evaluated. A significant decrease in body weight, higher values of kidney function tests, histopathological scores, and mtDNA deletion ratios were observed in group I compared to control group at days 7 and 30 (p < 0.05). In group II, there was a slight decrease in body weight at same days (p = 0.931 and 0.084, respectively). Kidney function tests, histopathological scores, and mtDNA common deletion ratios were statistically better in group II than group I at 7th and 30th day (p < 0.05). Although creatine significantly reversed kidney functions and pathological findings, this improvement was not sufficient to reach normal control group's results at days 7 and 30. In conclusion, the present study demonstrates that creatine administration is a promising adjuvant protective drug for reducing nephrotoxic effect of cisplatin.
Introduction
Cisplatin (cis-diamminedichloroplatinum II) is an anti-neoplastic agent, which is commonly used for treatment of solid tumors. Cisplatin treatment is restricted by its adverse effects such as nephrotoxicity, ototoxicity, and neurotoxicity. Major dose limiting factor of these is nephrotoxicity.Citation1 Cisplatin is mainly eliminated via kidneys and it is accumulated in both proximal and distal nephrons.Citation2 Since cisplatin tends to accumulate especially in mitochondria, renal proximal tubule cells seem to be more susceptible to cisplatin-induced cell damage.Citation3 Studies on this toxicity reflect that mitochondrial DNA damage has an important role for the occurrence of cisplatin nephrotoxicity.Citation2 Although previous studies have investigated the roles of different agents on cisplatin-induced nephrotoxicity, the effect of protective strategies on this issue has not yet been clearly determined.
Creatine (Cr) is a popular ergogenic supplement among athletes. Creatine has been reported to have anti-inflammatory, antioxidant effects and it has been shown that it acts as a free radical scavenger.Citation4–8 Besides this, recent reports supported that Cr has additional beneficial effects on various neurological, muscular, cardiovascular disorders, and aging.Citation9–14
The aim of this study is to investigate the possible protective effect of creatine therapy on cisplatin-induced nephrotoxicity.
Materials and methods
The experimental protocol of this study was approved by the Ethical Committee of Ondokuz Mayis University for Animal Research. In this study, 60 male Sprague–Dawley rats were purchased from the Ondokuz Mayis University Experimental Research Center. The rats were kept in standard environmental conditions in rat cages, and offered standard rat chow and tap water. Temperatures ranged from 20 to 24 °C and humidity ranged from 55 to 60%.
All animals were divided into three groups: Group I: Cisplatin (n = 20 rats) (Cisplatin, Orna, Istanbul, Turkey) (7 mg/kg intraperitoneal (i.p.) single dose), group II: Cisplatin + creatine (n = 20 rats) (Sigma-Aldrich Corp, St. Louis, MO) (7 mg/kg cisplatin i.p. single dose and 300 mg/kg creatine p.o. daily for 30 days starting on first day of cisplatin injection), group III: Control group (n = 20 rats) (Serum Physiologic, Polifarma, Corlu, Tekirdag, Turkey, 2.5 mL/kg i.p.).
Creatine was dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich Corp, St. Louis, MO) and equal amount of DMSO was given to other groups for achieving standardization. All rats' body weights were recorded weekly.
Sacrifications were performed on 10 rats from all groups at first week and at the end of first month. Rats were sacrificed using ether anesthesia and cervical dislocation. Blood samples were obtained by heart puncture for determining blood urea nitrogen (BUN) and serum creatinine levels. Both kidneys were rapidly excised. Left kidneys were allocated for histopathological evaluation. For right kidneys, first of all, renal capsules were removed and an incision on coronal axis is performed with a sterile razor blade. Renal cortexes of kidneys for mitochondrial deoxyribonucleic acid (mtDNA) isolation and blood samples were transferred immediately and stored at approximately −80 °C until analysis.
DNA isolation and determination of mtDNA copy numbers with deletions
DNA isolation
High Pure PCR Template Preparation Kit (Roche Diagnostics GmbH, Mannheim, Germany) was used for total DNA extraction from renal cortex. The yield of total DNA was determined by the spectrophotometric absorbance at 260 nm (IMPLEN NanoPhotometer, Essex, UK) and expressed in μg/mL.
The application of real-time quantitative PCR
Acidic ribosomal phosphoprotein gene (36B4) (GenBank accession No.: NW 047376.1) that has no pseudo-gene and is expressed as a single copy was chosen to determine the copy number of nuclear genome. Primers for the rat, 36B4 were designed using Light Cycler Probe Design Software 2.0 (Roche Diagnostics GmbH, Mannheim, Germany) (). D-loop region was selected to determine the number of mtDNA. Primer sequences of mtDNA deletion region and D-loop region (GenBank accession No.: X14848) (Nicklas et al.Citation15), are shown in .
Table 1. Sequence of PCR primers.
Light Cycler PCR system (Roche Diagnostics GmbH, Mannheim, Germany) and SYBR Green I fluorescent dye were used in the 36B4, D-loop, mtDNA4834 deletion PCR experiments. The quantitative PCR (QPCR) protocols of 36B4, D-loop, and mtDNA4834 deletion are shown in .
Table 2. QPCR protocols of 36B4, D-loop, mtDNA4834 deletion, and telomere.
Calculation of the mtDNA copy and mtDNA deletion frequency
The mtDNA copy numbers are calculated using 2Cp methods by the QPCR. The mtDNA copy numbers per cell (mtDNA/cell) is equivalent to the mtDNA copy number divided by the copy number of the 36B4 nuclear gene that has two-copies/diploid genome [mtDNA/cell = (2Cp(D-loop)/2Cp(36B4)) × 2].
The mtDNA4834 deletion frequency was calculated by the ratio of the amount of the common deletion copy number to the mtDNA copy number and multiplying the result with 100. [mtDNA4834 deletion frequency (%) = (2Cp(mtDNA4834 deletion)/2Cp(D-loop)) × 100].
Determination of BUN and creatinine levels
BUN and creatinine levels were measured by spectrophotometric method (Roche Diagnostics GmbH) with Cobas 800 (Roche) auto-analyzer. Levels were expressed in mg/dL.
Histological examination of the kidney
The left kidney was incised on coronal axis and fixed in 4% neutral formalin for 24 h. After fixation and tissue processing, the kidneys were embedded in paraffin block and then sectioned 4 μm. The sections were stained with periodic acid-Schiff (PAS) and evaluated with 200 × magnification objective (Olympus BX50 microscope, Olympus Optical Company, Tokyo, Japan). Tubulointerstitial injury, such as epithelial vacuolization, desquamation, necrosis, protein casts in dilated tubular lumens and tubular atrophy/interstitial fibrosis was semi-quantitatively assessed in whole corticomedullary region.Citation16 Injury score was on a scale of 0–4 based on the percentage of tubules affected (ranging from 0, normal; 1, < 10%; 2, 10–25%; 3, 26–75%; 4, > 75%) and an average was determined for each section. Without any knowledge of the experimental group, a pathologist made all evaluations on coded sections.
Statistical methods
Variables were analyzed using “SPSS for Windows© 15.0” (SPSS Inc., Chicago, IL) statistical software package. Data were presented as median (range). For comparisons, the Kruskal–Wallis analysis of variance and the Mann–Whitney U-test were used as appropriate. Values of p < 0.05 were considered significant.
Results
Creatine ameliorates cisplatin-induced weight loss and renal dysfunction
A significant decrease in body weight (BW) was observed in group I compared to control group at days 7 and 30 (p = <0.001 and <0.001, respectively), whereas there was a slight decrease in body weight in group II compared to group III at same those days () (p = 0.931 and 0.084, respectively).
Table 3. Weight alterations of rats through experiment.
In terms of kidney function tests, all groups' BUN and creatinine values were statistically different at day 7 and 30 of the experiment (). The highest values of these parameters were observed in group I. Kidney function tests in group II were better than group I at 7th and 30th day and group II levels were closer to group III rather than group I at 30th day.
Table 4. Kidney function tests alterations of rats through experiment.
Genotoxicity in cisplatin-treated rats was partially attenuated by creatine
To demonstrate the genotoxic effect of cisplatin, we evaluated the mtDNA common deletion ratio in treatment and control groups. At the end of first week, deletion rate in group I was higher than group II and III (p = 0.017 and <0.001, respectively). In addition, these differences retained until the 30th day of the experiment ().
Table 5. Common deletions alterations of rats through experiment.
Creatine treatment attenuates the histopathological damage scores
Histopathological scores were lower in group II than group I and higher than group III at days 7 and 30. In addition, although a slightly decreasing pattern of scores was observed in group II ( and ), normalization of scores were not observed in group I and II at 7th and 30th days.
Figure 1. Representative PAS-staining sections in each group. (a) Group I at 7th day, (b) Group II at 7th day, (c) Group III at 7th day, (d) Group I at 30th day, (e) Group II at 30th day, (f) Group III at 30th day (200 × magnification).
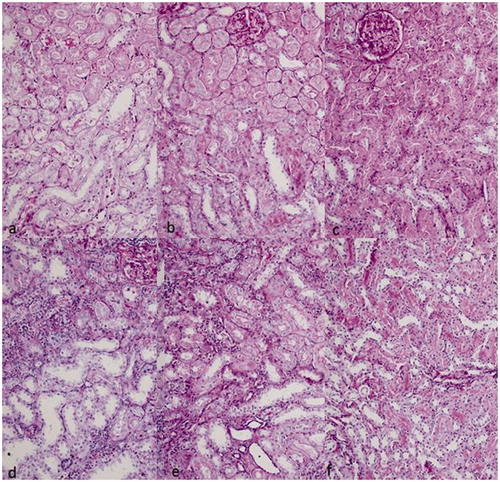
Table 6. Histopathological scores alterations of rats through experiment.
Discussion
This study was aimed to investigate the effect of creatine on cisplatin-induced nephrotoxicity in terms of serum BUN and creatinine levels, histopathological changes, mtDNA common deletion rates, and body weights of rats. With this study, it has been demonstrated that creatine supplementation attenuates the expected toxicity compromised by cisplatin.
Several mechanisms involving oxidative stress, DNA damage and apoptosis has been proposed to explain the mechanisms responsible for cisplatin-induced nephrotoxicity. Recent studies indicated the role of inflammation in cisplatin-induced nephrotoxicity and increased expression of TNF-α and other proinflammatory cytokines, such as IL-1β, IL-18, IL-6, has been previously reported.Citation2 There are also reports indicating that cisplatin inhibits fatty acid oxidation and mitochondrial complexes I to IV of the respiratory chain and induces decrease in intracellular ATP levels and cytotoxicity results in DNA damage.Citation2,Citation3,Citation17,Citation18 Cisplatin activates several apoptotic cascades and causes formation of reactive oxygen radicals.Citation2 Oxidative damage to mtDNA leads to a decrease in ATP production, loss of membrane potential, and eventually cell death. Since the mitochondrial dysfunction and damage has been shown to be one of the key mechanisms in cisplatin-induced nephrotoxicity, we assessed the role of creatine, an energy precursor, on mtDNA damage along with kidney function tests and histopathological examinations in our study. In this study, group I and II had higher mtDNA common deletion ratios than control group indicating that cisplatin leads to mitochondrial damage. Additionally, unchanged deletion values on the 30th day in each group might depend on insufficient time for regression of deletions.
Previous reports support that cisplatin-induced nephrotoxicity can be ameliorated by free radical scavengers, iron chelators, and several antioxidants.Citation19–22 Creatine has reported to have cytoprotective effects via stabilization of cellular membranes and maintenance of ATP.Citation23 In addition, anti-inflammatory activities of creatine on endothelial cells have been shown recently.Citation4 Berneburg et al.Citation5 observed that creatine supplementation normalized ultraviolet-A-irradiation induced mitochondrial mutagenesis and showed the link between mtDNA common deletion rate and energy metabolism. Several reports showed that creatine supplementation enhances oxidative phosphorylation and creatine has direct scavenger effects.Citation6–8 Guidi et al.Citation24 reported that creatine has a protective effect on oxidatively injured mtDNA. Sestili et al.Citation8 also reported cytoprotective effect of creatine on oxidatively injured cultured mammalian cells through direct antioxidant activity. We observed that rats given cisplatin alone had more weight loss, higher BUN and creatinine levels and higher histopathologic scores and mitochondrial DNA damage. Our results indicate that creatine administration has attenuated the toxic effect of cisplatin not only on biochemical and histopathologic scores, but also on mitochondrial damage. Since there is no study in the literature assessing the effect of creatine on cisplatin-induced nephrotoxicity, it was not possible to compare our results. Due to the fact that our study was not designed to explain the possible mechanism of creatine administration on the cisplatin-induced nephrotoxicity; we cannot draw a certain conclusion for the mechanisms. However, based on previous reports, the beneficial effect of creatine can be explained by preventing the decrease in intracellular energy pools and with own anti-inflammatory and antioxidant effect.
Various doses of creatine ranging from 143 mg/kg to 5 g/kg have been used in previous animal studies.Citation25–30 In this study, we gave creatine at the dose of 300 mg/kg, which is equal to a loading dose of 20 mg/kg in a 70 kg person and used in many of the animal studies.Citation27–30 Since there is no data regarding creatinine's effect on cisplatin nephrotoxicity, effective dose of creatine is not known yet. Therefore, the dose used in our study might be insufficient and this can be a limitation of our study. Further studies are required to evaluate the different doses of creatine in cisplatin toxicity.
Although creatine significantly attenuated the kidney function and mtDNA deletions in group II, this improvement was not sufficient to reach normal control group's results at day 30. This can be attributed to many reasons. Firstly, the dose of creatine used in the study could be insufficient. Secondly, 30 days might be a short period to get a complete improvement in mitochondrial damage. Finally, creatine could not have any effect on the other mechanisms rather than ATP depletion and oxidative damage.
In conclusion, administration of creatine seems to have a beneficial effect on cisplatin-induced nephrotoxicity as an adjuvant protective drug. Further studies are warranted to evaluate combination therapies with different doses of creatine or drugs that cover different pathways.
Declaration of interest
The authors report no conflicts of interest.
This study was supported by grants from the Ondokuz Mayis University Research Fund with the number PYO.TIP.1901.09.013.
References
- Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003;23(5):460–464
- Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of Cisplatin nephrotoxicity. Toxins (Basel). 2010;2(11):2490–2518
- Qian W, Nishikawa M, Haque AM, et al. Mitochondrial density determines the cellular sensitivity to cisplatin-induced cell death. Am J Physiol Cell Physiol. 2005;289:C1466–C1475
- Nomura A, Zhang M, Sakamoto T, et al. Anti-inflammatory activity of creatine supplementation in endothelial cells in vitro. Br J Pharmacol. 2003;139(4):715–720
- Berneburg M, Gremmel T, Kurten V, et al. Creatine supplementation normalizes mutagenesis of mitochondrial DNA as well as functional consequences. J Invest Dermatol. 2005;125:213–220
- Rico-Sanz J. Creatine reduces human muscle PCr and pH decrements and P(i) accumulation during low-intensity exercise. J Appl Physiol. 2000;88:1181–1191
- Walsh B, Tonkonogi M, Soderlund K, Hultman E, Saks V, Sahlin K. The role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. J Physiol. 2001;537(Pt 3):971–978
- Sestili P, Martinelli C, Bravi G, et al. Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic Biol Med. 2006;40(5):837–849
- Tarnopolsky MA. Creatine as a therapeutic strategy for myopathies. Amino Acids. 2011;40(5):1397–1407
- Matthews RT, Ferrante RJ, Klivenyi P, et al. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp Neurol. 1999;157(1):142–149
- Easton C, Turner S, Pitsiladis YP. Creatine and glycerol hyperhydration in trained subjects before exercise in the heat. Int J Sport Nutr Exerc Metab. 2007;17:70–91
- Beis LY, Polyviou T, Malkova D, Pitsiladis YP. The effects of creatine and glycerol hyperhydration on running economy in well trained endurance runners. J Int Soc Sports Nutr. 2011;8(1):24
- Berneburg M, Gremmel T, Kürten V, et al. Creatine supplementation normalizes mutagenesis of mitochondrial DNA as well as functional consequences. J Invest Dermatol. 2005;125(2):213–220
- Bender A, Beckers J, Schneider I, et al. Creatine improves health and survival of mice. Neurobiol Aging. 2008;29(9):1404–1411
- Nicklas JA, Brooks EM, Hunter TC, Single R, Branda RF. Development of a quantitative PCR (TaqMan) assay for relative mitochondrial DNA copy number and the common mitochondrial DNA deletion in the rat. Environ Molec Mutagenesis 2004;44:313–320
- Ozkaya O, Yavuz O, Can B, et al. Effect of rosiglitazone on cisplatin-induced nephrotoxicity. Ren Fail. 2010;32(3):368–371
- Li S, Wu P, Yarlagadda P, et al. PPAR alpha ligand protects during cisplatin-induced acute renal failure by preventing inhibition of renal FAO and PDC activity. Am J Physiol Renal Physiol. 2004;286:F572–F580
- Kruidering M, Van de Water B, de Heer E, Mulder GJ, Nagelkerke JF. Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: mitochondrial dysfunction by inhibition of complexes I to IV of the respiratory chain. J Pharmacol Exp Ther. 1997;280:638–649
- Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV. In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int. 1998;53:394–401
- Davis C, Nick H, Agarwal A. Manganese superoxide dismutase attenuates cisplatin-induced renal injury: Importance of superoxide. J Am Soc Nephrol. 2001;12:2683–2690
- Naziroglu M, Karaoglu A, Aksoy AO. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology. 2004;195:221–230
- Kroning R, Lichtenstein AK, Nagami GT. Sulfur-containing amino acids decrease cisplatin cytotoxicity and uptake in renal tubule epithelial cell lines. Cancer Chemother Pharmacol. 2000;45:43–49
- Persky AM, Brazeau GA. Clinical pharmacology of dietary supplement Cr monohydrate. Pharmacol Rev. 2001;53:161–176
- Guidi C, Potenza L, Sestili P, et al. Differential effect of creatine on oxidatively-injured mitochondrial and nuclear DNA. Biochim Biophys Acta. 2008;1780(1):16–26
- Franco FS, Costa NM, Ferreira SA, Carneiro-Junior MA, Natali AJ. The effects of a high dosage of creatine and caffeine supplementation on the lean body mass composition of rats submitted to vertical jumping training. J Int Soc Sports Nutr. 2011;8:3
- McGuire M, Bradford A, MacDermott M. The effects of dietary creatine supplements on the contractile properties of rat soleus and extensor digitorum longus muscles. Exp Physiol. 2001;86(2):185–190
- Young RE, Young JC. The effect of creatine supplementation on mass and performance of rat skeletal muscle. Life Sci. 2007;81(9):710–716
- Young JC, Young RE. The effect of creatine supplementation on glucose uptake in rat skeletal muscle. Life Sci. 2002;71(15):1731–1737
- de Souza RA, Xavier M, da Silva FF, et al. Influence of creatine supplementation on bone quality in the ovariectomized rat model: an FT-Raman spectroscopy study. Lasers Med Sci. 2012;27(2):487–495
- Ozkan O, Duman O, Haspolat S, et al. Effect of systemic creatine monohydrate supplementation on denervated muscle during reinnervation: experimental study in the rat. J Reconstr Microsurg. 2005;21(8):573–579

