Abstract
The presence of granular swollen epithelial cells (GSECs) in tubular cells was recently reported to be a specific change associated with mitochondrial cytopathy. However, at present, GSEC is not routinely evaluated. We, in this study, present a case of glomerulosclerosis, in which the presence of GSECs should provide us one clue to understand the pathogenesis of its progressive decline of renal function. A 54-year-old Japanese female, who had been diagnosed with Graves’ disease, was referred for the examination and treatment of her proteinuria (5.4 g/gCre at the first visit to our hospital). A kidney biopsy showed 28.6% of the glomeruli to be globally sclerosed and 10.7% of the glomeruli to have completely collapsed. However, according to a light microscopic analysis, all other glomeruli showed an almost normal appearance, except for some slight enlargement. Almost 30% of the interstitium was damaged by fibrosis. Characteristically, GSECs were observed in the medulla collecting ducts. Although she had no symptoms of either myopathy or encephalopathy, no history of stroke-like episodes or difficulty in hearing, her serum concentrations of lactate and pyruvate were both elevated. Therefore, mitochondrial DNA sequencing was performed to assess the etiopathogenesis of her nephropathy. Consequently, a homoplasmic 7501 T > A replacement, which has not been previously reported in patients with renal diseases, was detected. This case suggests that the routine evaluation of GSECs can provide important clues to assess the etiopathogenesis of cryptogenic glomerulosclerosis.
Introduction
The 3243 A > G mutation is one of the most frequently observed mutations of mitochondrial DNA (mtDNA). In the past two decades, many reports have described that the 3243 A to G mutation could cause focal segmental sclerosis (FSGS).Citation1–3 By electron microscopy, some podocytes were found to have an increased number of enlarged mitochondria in their cytoplasm. It was recently reported that patients with mitochondrial cytopathy have pathological changes not only in glomeruli but also in tubules. The characteristic pathological cells were designated as granular swollen epithelial cells (GSECs), because the cells include increased and enlarged mitochondria in their cytoplasm, which makes them look “swollen”.Citation4
We herein present a case of glomerulosclerosis accompanied with GSECs, in whom a new mtDNA variant, which has not been previously reported in patients with renal diseases, was detected.
Case
This case was a 54-year-old Japanese female, who was referred to Chiba-East Hospital by her primary doctor in order to examine and treat her proteinuria. When she was 50 years old, cardiomegaly had been pointed out by the chest X-ray taken during an annual health check-up, although she had no symptoms. She visited her primary doctor and was diagnosed to have Basedow’s disease (thyroid-stimulating hormone; TSH < 0.002 µIU/mL, FT3 6.0 pg/mL, FT4 2.4 ng/mL, TSH receptor antibody 50.5%) and atrial fibrillation (Af). Therefore, she had been prescribed propylthiouracil (PTU) and digoxin. At 52 years of age, her blood glucose level was elevated, and she was diagnosed with diabetes mellitus (DM). Thereafter, she had also been taking a dipeptidyl peptidase 4 inhibitor. Beginning when she was 53 years old, the patient’s urinary examination by the test tape started to show proteinuria from 1+ to 3+. She had also been prescribed an angiotensin II receptor blocker to treat hypertension for three months until the first visit to Chiba-East Hospital. At the first visit, her blood pressure was 157/117 and the proteinuria was 5.4 g/gCre, although the serum albumin level was 4 mg/dL. She had moderate pretibial pitting edema (PTPE).
Because she reported consuming a lot of salt, she was recommended to start a low salt diet (under 6 g/day of salt). One month after the first visit to our hospital, she was admitted in order to undergo examinations due to proteinuria. At this admission, her blood pressure was 139/93 without any change in the drugs being administered at the first visit, and her PTPE had disappeared. The proteinuria also had decreased to 1.5 g/gCre. A physical examination showed a height of 153.1 cm, body weight of 54.1 kg and body mass index of 23.1. No crackles or murmurs were detected on chest auscultation. Although she had been suffering from DM for two years, no evidence of diabetic neuropathy was observed. No skin lesions were detected. An ophthalmologic examination detected neither diabetic retinopathy nor hypertensive retinopathy. Thyroid enlargement was also not detected by palpation.
The patient’s birth weight had been 3500 g at 40 weeks of gestation. Her mother suffered from DM, and her father suffered from a kidney disease whose etiology had not been assessed. She was not a smoker. Laboratory tests on admission () showed slightly elevated serum creatinine and positive myeloperoxidase-anti-neutrophil cytoplasmic antibody (MPO-ANCA). However, no signs of vasculitis were detected. We concluded that PTU likely triggered this MPO elevation.Citation5
Table 1. The findings of laboratory tests performed on admission.
Electrocardiography showed Af (heart rate: 51 bpm) and moderate down-sloping ST depression and inverted T waves on leads I, II, aVF, V4-6, which could be explained by the digitalis effect. On chest X-rays, although severe cardiomegaly (cardiothoracic ratio, 67.1%) was detected, there was neither enlargement of the pulmonary artery shadow nor pleural effusion. An echogram of the thyroid revealed no swelling on either side and no masses. We performed a percutaneous renal biopsy under ultrasound guidance two days after admission to investigate the etiology of the heavy proteinuria.
The light microscopic photos of the kidney biopsy specimen are shown in . We obtained 28 glomeruli. There were eight glomeruli (28.6%) with global sclerosis and three glomeruli (10.7%) whose tufts were completely collapsed. However, all other glomeruli showed an almost normal appearance without any sclerotic lesions, tuft adhesions, endocapillary proliferation or crescent formations. Slight enlargement of all glomeruli was detected. Almost 30% of the area of the interstitium was damaged with fibrosis, but there were no inflammatory cells. Interestingly, GSECs were observed in the medulla collecting ducts (). The arteriole endothelium showed slight hyalinosis, and the vascular smooth muscle cells of the arterioles were enlarged. Endothelial hyalinosis was previously reported as a pathological finding of mitochondria cytopathy. An electron microscopic analysis () showed moderate foot process effacement and moderate thickening of glomerular basement membrane. In all podocytes observed, there were no increases in the numbers of mitochondria in the cytoplasm. However, we found an increase number of enlarged mitochondria in the tubular cells.
Figure 1. The light microscopic findings. (A) While globally sclerosed glomeruli and collapsed glomeruli were observed, the other glomeruli showed an almost normal appearance (Masson trichrome stain, original magnification ×40). (B) This glomerulus looks normal. However, the vascular smooth muscle cells are disorganized and enlarged (PAM-HE stain, original magnification ×200). (C) This glomerulus also looks normal, while the myocytes of the afferent arteriole are enlarged and their endothelium has slight hyalinosis (PAM-HE stain, original magnification ×200). (D) Granular swollen epithelial cells are indicated by arrows (Masson trichrome stain, original magnification ×400).
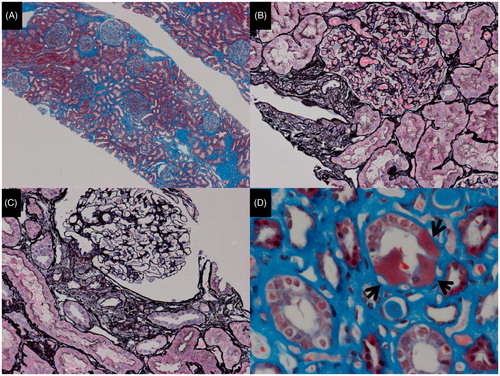
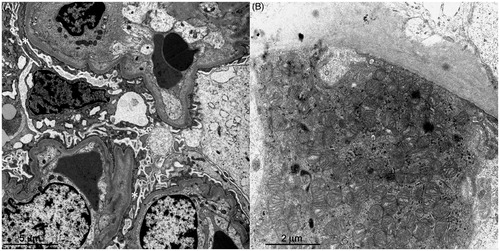
Figure 2. The electron microscopic findings. (A) The foot processes of podocytes are effaced (asterisks). (B) The granular swollen epithelial cells have increased numbers of mitochondria, a portion of which are enlarged, and some of which have cristae that have lost their normal structure.
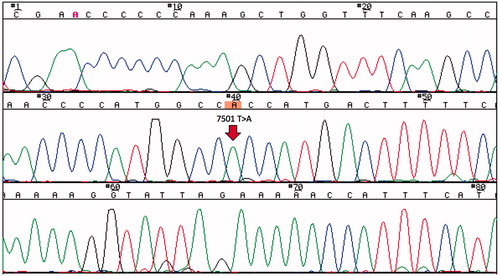
As GSEC was previously reported to be a specific change associated with mitochondrial cytopathy, we measured serum lactate and pyruvate concentrations. These serum concentrations were elevated at 22.9 mg/dL (normal: 3.0–17.0) and 2.10 mg/dL (normal: 0.30–0.94), respectively. Therefore, we suspected a mtDNA mutation and examined the patient’s blood cells by direct sequencing after obtaining both permission from our ethics committee and informed consent from the patient. As a result, a homoplasmic 7501 T > A replacement was detected (). She had no symptoms of myopathy or encephalopathy, no history of stroke-like episodes or difficulty in hearing.
Figure 3. The results of the direct sequencing of mtDNA. A homoplasmic 7501 T > A variant in the tRNASer(UCN) was detected in the patient’s blood sample.
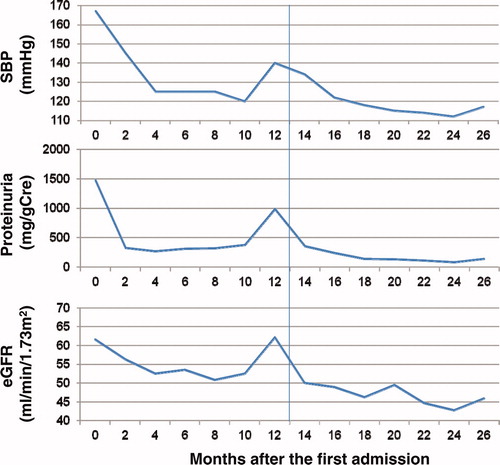
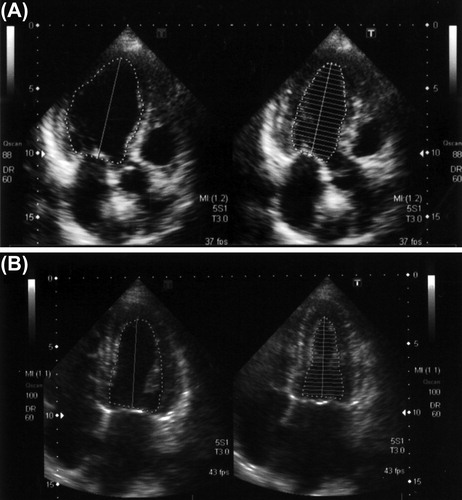
The echographic examination for her heart showed hypokinesis in the anteroseptum (, the ejection fraction by the modified Simpson’s method was 45.9%). Therefore, she underwent coronary angiography. However, there were no abnormalities in her coronary arteries. Thereafter, we added a β-blocker, bisoprolol fumarate. After the prescription, the findings of cardiac echography changed to normal (ejection fraction by modified Simpson’s method: 60.7%). Furthermore, the ST depression and inverted T waves on leads I, II, aVF, V4-6 recovered, although digitalis was given at same dose as at the first admission. Her proteinuria also decreased after the β-blocker, which was accompanied by a decrease in blood pressure (). However, her estimated glomerular filtration rate has been gradually decreasing for the past two years. Although the MPO-ANCA level was elevated at the first admission to our hospital, there has been no evidence of vasculitis (two years after the first admission).
Figure 4. The B-mode echocardiogram. (A) The cardiac echogram on admission showed hypokinetic wall motion and anteroseptal hypokinesis. The ejection fraction determined by the modified Simpson’s method was 45.9%. (B) One year after the prescription of a β-blocker, the cardiac echogram showed normal wall motion of the anteroseptum. The ejection fraction determined by a modified Simpson’s method was 60.7%.
Discussion
Although we could neither find any FSGS lesions nor increased mitochondria in her podocytes, the renal pathology of this case showed similar changes to those of the nephropathy in patients with mitochondrial cytopathy in the following three points: progressive renal damage, abnormality of myocytes in the afferent arterioles and small arteries, and especially, the existence of GSECs in the tubules.Citation1–3 In addition, the concentrations of serum lactate and pyruvate were elevated. Therefore, we analyzed the patient for mtDNA mutations and consequently detected a homoplasmic 7501 T > A mtDNA variant.
It was reported that gene mutations of COQ2Citation6 and COQ6,Citation7 which are essential for COQ10 synthesis, cause FSGS. Coenzyme Q10 is an essential cofactor of the electron transport chain in the mitochondrial membrane. Therefore, mitochondrial function should be involved in glomerular podocyte function.Citation8 Furthermore, case reports of mtDNA mutations and deletions not only in FSGS but also in tubulointerstitial nephritis and tubulopathies have been gradually accumulating.Citation9 These reports indicate that mitochondria are likely involved in the pathogenesis of a considerable number of renal diseases, to a much greater extent than was considered previously.
In this study, a homoplasmic 7501 T > A variant in the tRNASer(UCN) was detected in our case of glomerulosclerosis with GSECs. This 7501 T > A replacement is currently classified as a normal variant in the MitoMap database (http://www.mitomap.org/MITOMAP). However, 7501 T > A should modify the secondary structure of the D-arm in the tRNASer(UCN) transcript.Citation10 The D-arm plays important roles in the stability of the transcript and the general rate of mitochondrial protein synthesis.Citation11 Furthermore, the 7501 T > A variant has been reported in patients with hearing loss. The 7501 T > C variant, which is also classified as a normal variant, was reported in a cardiomyopathy patient.Citation12 We again emphasize that it is not evident that the mitochondrial 7501 T > A variant truly has pathogenicity, and further tests, including functional studies, family studies and population studies are needed to verify its effects.
We propose another possible explanation for why the 7501 T > A variant causes glomerulosclerosis. As described above, the D-arm of the tRNASer(UCN) transcript is important for the general rate of mitochondrial protein synthesis. Thyroid hormone stimulates mitochondrial activity.Citation13 In this case, the patient also had Basedow’s disease. Such a situation might make this mtDNA variant pathogenic, although it may not normally be pathogenic in healthy individuals. Interestingly, the patient’s cardiac echogram showed hypokinesis in the anteroseptum wall. However, after the prescription of a β-blocker, the motion of the anteroseptum wall recovered. Therefore, although we cannot confirm the diagnosis of cardiomyopathy in this case, mitochondrial dysfunction as result of the 7501 T > A variant might be able to cause cardiomyopathy similar to the 7501 T > C variant.Citation12
In this case, the first and the strongest reason why we suspected the involvement of mitochondria was the pathological finding of GSECs, even without any signs of mitochondrial cytopathy and without FSGS and an increase in the number of mitochondria in glomerular podocytes, which is characteristic of mtDNA mutations. Previously, GSECs have not been evaluated in routine clinical tests. This case suggests that GSECs can provide a clue to assess the etiopathogenesis of cryptogenic glomerulosclerosis and tubulopathy.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by the JSPS (Japan Society for the Promotion Science) KAKENHI Grant Number 80348276 to T. Imasawa, and a grant from the National Hospital Organization of Japan to T. I.
References
- Doleris LM, Hill GS, Chedin P, et al. Focal segmental glomerulosclerosis associated with mitochondrial cytopathy. Kidney Int. 2000;58:1851–1858
- Hotta O, Inoue CN, Miyabayashi S, et al. Clinical and pathologic features of focal segmental glomerulosclerosis with mitochondrial tRNALeu(UUR) gene mutation. Kidney Int. 2001;59:1236–1243
- Guéry B, Choukroun G, Noël LH, et al. The spectrum of systemic involvement in adults presenting with renal lesion and mitochondrial tRNA(Leu) gene mutation. J Am Soc Nephrol. 2003;14:2099–2108
- Kobayashi A, Goto Y, Nagata M, Yamaguchi Y. Granular swollen epithelial cells: A histologic and diagnostic marker for mitochondrial nephropathy. Am J Surg Pathol. 2010;34:262–270
- Ishii R, Imaizumi M, Ide A, et al. A long-term follow-up of serum myeloperoxidase antineutrophil cytoplasmic antibodies (MPO-ANCA) in patients with Graves disease treated with propylthiouracil. Endocr J. 2010;57:73–79
- Diomedi-Camassei F, Di Giandomenico S, Santorelli FM, et al. COQ2 nephropathy: A newly described inherited mitochondriopathy with primary renal involvement. J Am Soc Nephrol. 2007;18:2773–2780
- Heeringa SF, Chernin G, Chaki M, et al. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J Clin Invest. 2011;121:2013–2024
- Imasawa T, Rossignol R. Podocyte energy metabolism and glomerular diseases. Int J Biochem Cell Biol. 2013;45:2109–2118
- O'Toole JF. Renal manifestations of genetic mitochondrial disease. Int J Nephrol Renovasc Dis. 2014;7:57–67
- Mutai H, Kouike H, Teruya E, et al. Systematic analysis of mitochondrial genes associated with hearing loss in the Japanese population: dHPLC reveals a new candidate mutation. BMC Med Genet. 2011;12:135
- Möllers M, Maniura-Weber K, Kiseljakovic E, et al. A new mechanism for mtDNA pathogenesis: impairment of post-transcriptional maturation leads to severe depletion of mitochondrial tRNASer(UCN) caused by T7512C and G7497A point mutations. Nucleic Acids Res. 2005;33:5647–5658
- Zaragoza MV, Fass J, Diegoli M, Lin D, Arbustini E. Mitochondrial DNA variant discovery and evaluation in human cardiomyopathies through next-generation sequencing. PLoS One. 2010;5:e12295
- Wrutniak-Cabello C, Casas F, Cabello G. Thyroid hormone action in mitochondria. J Mol Endocrinol. 2001;26:67–77