Abstract
Objectives: To analyze the effect of treating metabolic syndrome (MetS) on further kidney function decline in patients with early-stage chronic kidney disease (CKD). Methods: In a study period of 24 months, 162 patients with early stage CKD were enrolled. Baseline and follow-up data related to the occurrence of MetS and glomerular filtration rate (GFR) were assessed. Subjects were classified into controlled MetS (group 1) and uncontrolled MetS (group 2). Furthermore, they were subdivided into four subgroups: (A) controlled MetS at baseline and at follow-up, (B) uncontrolled MetS at baseline but controlled MetS at follow-up visits, (C) controlled MetS at baseline but uncontrolled MetS at follow-up visits, and (D) uncontrolled MetS at baseline and follow-up visits. Results: Final GFR was lower in group 2 versus group 1 (69.21 ± 20.20 vs. 82.86 ± 22.33 mL/min/1.73 m2, p <0.001). The presence of MetS had high risk to develop late-stage CKD (HR = 3.279, 95% CI: 1.545–6.958, p = 0.002). Moreover, subgroup D (HR = 2.982, 95% CI: 1.287–6.908, p = 0.011) and the presence of three (p = 0.026) or four (p = 0.049) metabolic components had high risk to develop late-stage CKD. Conclusion: Treating MetS slows CKD progression in patients with early-stage of CKD.
Introduction
Despite recent advances in the treatment of chronic kidney disease (CKD), it remains an important public health challenge.Citation1 More and more studies have begun to focus on the pre-CKD stage.Citation2 Metabolic syndrome (MetS), characterized by abdominal obesity, hypertriglyceridemia, decreased high-density lipoprotein cholesterol, hypertension and hyperglycemia,Citation3 is an important issue in modern west society because of its association between the development of diabetes mellitus and cardiovascular disease as well as all-cause mortality.Citation4,Citation5 The association between MetS and CKD was recently analyzed.Citation6 MetS has a variety of risk factors for CKD, such as insulin resistance, hypertension, abdominal obesity and dyslipidemia.Citation7 Luk et al. established that the impact of MetS on CKD progression might be prominent in early stage of diabetic or non-diabetic patients.Citation8 We discussed the goal in treating MetS to prevent or slow further damage of renal function, and the study presented a framework to consider the likelihood of progression from early stage to late stage of CKD. The prevalence of MetS in males and females is approximately 11.2 and 18.6% in Taiwan according to National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III), respectively.Citation9–11 Moreover, it has been recognized as inflammatory and thrombotic situations where inflammatory cytokines may cause renal injury.Citation12,Citation13 There was a steep rise of renal function decline in patients with MetS, and the aggressive screening and management might be warranted in those patients.Citation14 The incidence of CKD and the risk of CKD progression are increased based on the parameters of MetS criteria.Citation15 Nevertheless, a link between the treatment of MetS and CKD progression is still unclear.
Previous published data demonstrated that MetS lost its predictive value in late-stage CKD progression so the benefits for efficient correction of MetS in late-stage CKD patients might be marginal.Citation16 Therefore, we aimed our research to analyze the risk of kidney function deterioration in early-stage CKD between the groups of controlled MetS and uncontrolled MetS.
Materials and methods
Participants
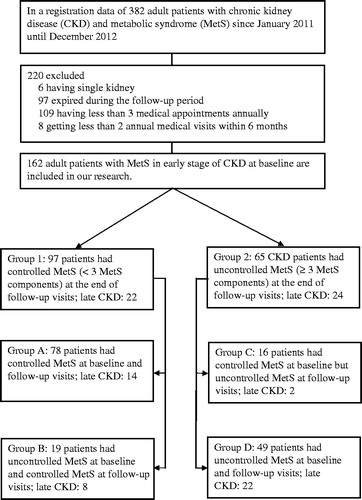
This retrospective comparative cohort study was conducted in Tao Yuan General Hospital, Ministry of Health and Welfare. Data were collected from early stage of 382 CKD patients with MetS, older than 18 years of age from January 2011 to December 2012. 220 patients with single kidney, death during the follow-up period, annual medical visits less than three times, or medical appointments less than two times within 6 months were excluded. Of these, 162 CKD patients met the inclusion criteria (). Participants were recruited by follow-up visits. All MetS participants signed their written informed consent. This study was approved by the Institutional Review Board of Tao Yuan General Hospital, Ministry of Health and Welfare. Our research was performed fulfilling the principles of Helsinki Declaration and the International Guidelines for Ethical Review for Epidemiological Studies.
Study measurements
The variables including date of birth, date of follow-up, age, and gender were recorded in electrical medical chart. Sociodemographic characteristics including personal health history (coronary artery disease, stroke, hypertension, and diabetes) were obtained by questionnaire. Body weight, height, and blood pressure were measured with standardized protocol at the morning between 08:00 and 11:00 o’clock. Waist circumference was measured at the smallest circumference between the ribs and iliac crest, and hip circumference at maximum circumference between the iliac crest and crotch to the nearest 0.1 cm. One nurse measured each participant’s blood pressure (BP) for five times by sphygmomanometer after the subjects had rested for at least 5 min in the sitting position. The first and fourth Korotkoff sounds were used to represent the systolic and diastolic blood pressure. The average value of these five measuring points for systolic and diastolic blood pressure was recorded. Blood samples were drawn after overnight fasting for the measurement of plasma glucose concentration, serum concentrations of glycosylated hemoglobin (HbA1C), total cholesterol, high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), triglyceride (TG), hemoglobin, blood urine nitrogen, and creatinine. Fresh spot urine samples were collected for the measurement of urinary protein and urinary creatinine.
Determination of MetS
MetS was diagnosed according to the definition of Health Promotion Administration Ministry of Health and Welfare, Republic of China (Taiwan)Citation17 and National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III,Citation11 in addition to three or more of five factors. These categories are: waist circumference ≥90 cm for men and ≥80 cm for women or body mass index ≥27; triglycerides ≥150 mg/dL; systolic blood pressure (BP) ≥130 mmHg or diastolic BP ≥85 mmHg; fasting glucose ≥100 mg/dL; and HDL-c <40 mg/dL in men and <50 mg/dL in women.
Determination of CKD
Early stage of CKD was defined according to National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) Guidelines with estimated glomerular filtration rate (eGFR) more than 60 mL/min/1.73 m2. The development of late stage of CKD was eGFR less than 60 mL/min/1.73 m2. Baseline and follow-up eGFR were estimated according to the equation Modification Diet Renal Disease: Estimated GFR = 186 × serum creatinin−1.154 × age−0.203 × 1.21 (if black) × 0.742 (if female). Rapid kidney function decline (RKFD) was defined as a >5% annual eGFR decline from baseline.
Classification of MetS in CKD
Subjects with CKD in the occurrence of less than three normalized components of MetS were classified as group 1 [controlled metabolic syndrome (CMS)], and subjects with CKD in three or more abnormal levels of MetS components during their follow-up were classified as group 2 [uncontrolled metabolic syndrome (UMS)]. Both groups 1 and 2 were divided into total four categories. For group 1, the participants were divided into two subgroups: subgroup A as controlled MetS at baseline and follow-up visits; subgroup B as uncontrolled MetS at baseline but controlled MetS at follow-up visits. For group 2, we categorized the participants into two subgroups: subgroup C as MetS less than three components at the beginning but uncontrolled MetS at follow-up visits; subgroup D as uncontrolled MetS at baseline and follow-up visits. In order to further clarify the relationship of medical treatment between CMS and UMS, we examined our data with cumulative population receiving medications in different groups. The main outcome was the decrease in the eGFR in mL/min/1.73 m2 and the development of late-stage CKD (eGFR <60 mL/min/1.73 m2).
Statistical analysis
Continuous variables were summarized as mean (standard deviation) unless otherwise stated. Student’s t test, Chi-squared, or Mann–Whitney U test was used to assess differences of the demographic data, laboratory variable between groups 1 and 2. The Kruskall–Wallis test was used for the comparison of final eGFR according to the number of MetS components.
Logistic regression models were conducted to identify independent variables associated with the development of late-stage CKD among groups 1, 2, subgroups A, B, C, and D. In the logistic regression, we included potential confounders in the model. The first model was used to evaluate the impact of group 2 at follow-up. The second model evaluated the risk for the development of late-stage CKD according to subgroup B, subgroups C and D at the follow-up. Subgroup A was used as the role of our reference. The third model evaluated the development of late-stage CKD according to the number of MetS components. The fourth model assessed the impaction of each component of MetS for the development of late-stage CKD. A p value of less than 0.05 was considered statistically significant. The log-rank test was used to confirm the difference in the period to develop late stage of CKD among those groups. All statistical analyses were performed using SPSS software (version 17.0, SPSS Inc., Chicago, IL).
Results
Over the 24-month period, 162 Taiwanese patients with stage 1 or stage 2 CKD were included in the study. Eighty-five were man (52.2%). Their median age was 65.53 years (range 18–93 years). Ninety-seven participants (59.9%) were classified as CMS (group 1: subgroup A, n = 78; subgroup B, n = 19) and 65 (40.1%) people were classified as UMS (group 2: subgroup C, n = 16; subgroup D, n = 49). The baseline demographic and biochemical data were described in and . At baseline, age, gender, total cholesterol, systolic and diastolic blood pressure, baseline eGFR, serum creatinine and albumin did not reveal any difference between UMS and CMS. Triglycerides (108.51 ± 60.61 vs. 203.49 ± 152.96 mg/dL, p < 0.001), HDL-c (49.04 ± 16.22 vs. 38.93 ± 1.76 mg/dL, p < 0.001), fasting blood glucose (110.89 ± 34.83 vs. 139.13 ± 46.81 mg/dL, p < 0.001), body mass index (24.20 ± 3.88 vs. 28.99 ± 4.47 kg/m2, p < 0.001), HbA1c (6.32 ± 1.44 vs. 7.00 ± 1.29%, p = 0.003), and those criteria for MetS (1.74 ± 1.31 vs. 3.38 ± 0.96, p < 0.001) were significantly different between two groups ().
Table 1. Baseline characteristics of chronic kidney disease patients with MetS (N = 162).
Table 2. Demographic and biochemical data during follow-up visits.
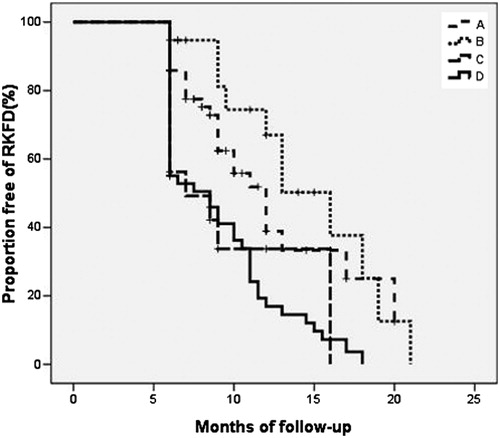
At the end of follow-up, the duration of follow-up, total cholesterol, LDL-c, fasting blood glucose, and urine protein-to-creatinine ratio did not differ between two groups. However, triglycerides (100.01 ± 51.50 vs. 207.20 ± 119.00 mg/dL, p < 0.001), body mass index (24.20 ± 4.01 vs. 29.08 ± 4.37 kg/m2, p < 0.001), systolic blood pressure (121.73 ± 17.11 vs. 132.03 ± 17.05 mmHg, p < 0.001), HbA1c (6.20 ± 1.21 vs. 7.14 ± 1.28%, p < 0.001), and the number of MetS components (1.30 ± 0.74 vs. 3.67 ± 0.70 components, p < 0.001) in CMS group were significantly lower than UMS group (). Besides, proportions of associated diabetes (39.2 vs. 66.1%, p = 0.001), hypertension (52.6 vs. 70.7%, p = 0.023), and decline of GFR (delta GFR1-GFR2) (5.58 ± 16.02 vs. 12.11 ± 13.19 mL/min/1.73 m2, p = 0.007) were significantly lower in CMS than UMS (). HDL-c (52.72 ± 16.09 vs. 39.22 ± 9.94 mg/dL, p < 0.001) and eGFR at the end of follow-up (eGFR2) (82.86 ± 22.33 vs. 69.21 ± 20.20 mL/min/1.73 m2, p < 0.001) were higher in CMS than UMS (). The duration of the progression to late-stage CKD in CMS group was later than UMS group (12.70 ± 4.36 vs. 10.45 ± 3.93 months, p = 0.049; ), but the difference was not observed in four subgroups (p = 0.051; ).
Figure 2. Proportions of free progression to late stage chronic kidney disease (eGFR < 60 mL/min/1.73 m2) in the group of controlled MetS and the group of uncontrolled MetS. Rapid kidney function decline (RKFD) was defined as a >5% annual eGFR decline from baseline.
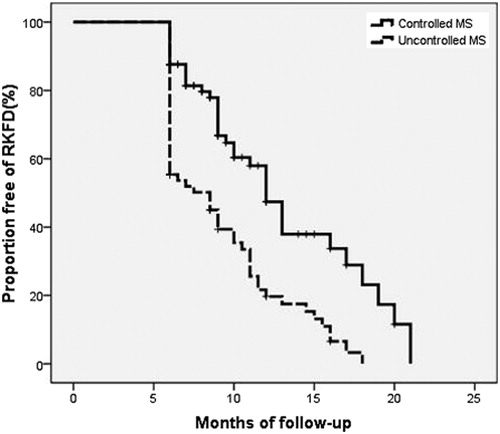
Figure 3. Proportions of free progression to late stage chronic kidney disease (eGFR < 60 mL/min/1.73 m2) in subgroups A, B, C and D. Rapid kidney function decline (RKFD) was defined as a >5% annual eGFR decline from baseline.
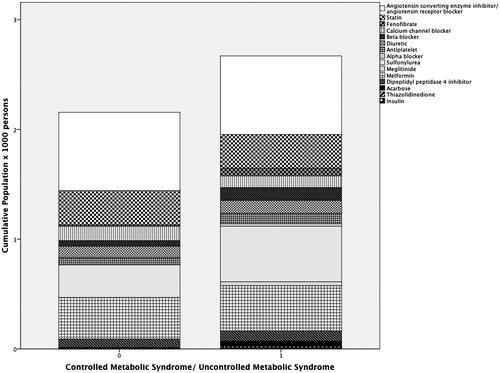
After conducted therapy among 162 tested subjects in , the most frequent medication in the study was angiotensin converting enzyme inhibitor/angiotensin receptor blocker in CMS and UMS. The second common medication was metformin in CMS. Sulfonylurea took second place along with those medications of UMS. The combination therapy with hypertension and diabetes mellitus is related to higher cumulative population regarding CKD patients with either CMS or UMS.
After adjusting age, gender, hypertension, proteinuria, obesity (body mass index), HbA1c and underlying disease, the occurrence of MetS was associated with higher risk for the development of late-stage CKD (HR = 3.279, 95% CI: 1.545–6.958, p = 0.002). Furthermore, the subgroup D had higher risk to develop late-stage CKD (HR = 2.982, 95% CI: 1.287–6.908, p = 0.011) and subgroup C showed the tendency to develop late-stage CKD (HR = 2.552, 95% CI: 0.953–6.832, p = 0.062) (). After adjusting all parameters in , patients with three (p = 0.026) or four (p = 0.049) MetS components had higher risk to develop late-stage CKD than those with less than three components. The strong association between the number of MetS components and renal function decline was confirmed. Although all components of MetS had similar impacts on the deterioration of renal function, hypertension demonstrated the tendency to have greater risk to develop late-stage CKD (HR = 1.744, 95% CI: = 0.917–3.493, p = 0.059) than other components of MetS.
Table 3. Logistic regression models to identify independent variables associated with the development of late-stage chronic kidney disease.
Discussion
Our retrospective observational cohort study established a positive and significant correlation of MetS and CKD between CMS and UMS groups. Subgroup D (the persistence of MetS ≥3 components at baseline and follow-up) had greater risk in the progression to late-stage CKD than other subgroups. Treating MetS delays the exacerbation of kidney function in early-stage of CKD. Besides, MetS independently associated with higher risk of CKD progression and increased numbers of metabolic components had higher risk of renal function deterioration. It should be emphasized that hypertension represented greater tendency to renal function decline. Some studies also discussed that diabetic patients with UMS were associated with decreased GFR and stage-to-stage change in comparison to those with normal levels of parameters in MetS.Citation18 Although the number of MetS components was incapable of detecting the development of declined GFR, those parameters were demonstrated to precede the tendency of decreased GFR. These findings imply that successful management of MetS can attenuate renal damage.Citation18 The proposed mechanisms to explain renal damage induced by MetS are hyperfiltration, proinflammatory and prothrombotic state, endothelial dysfunction, and oxidative stress.Citation12,Citation13,Citation19,Citation20 To circumvent these issues, MetS had been identified as initiating factor of CKD and had the impact on renal function progression in diabetic or non-diabetic early-stage CKD.Citation6,Citation8,Citation16 A sizeable study utilizing data from the Atherosclerosis Risk in Communities cohort confirmed a step-wise increase with the risk of developing CKD, each MetS criterion met by non-diabetic adults, even after putting in place prophylactic measures for the possible development of diabetes mellitus and hypertension.Citation15 In 2007, Tozawa et al. conducted a prospective study to examine MetS as a risk factor for new onset of CKD in 6371 subjects without CKD or diabetes mellitus at baseline in Japan. The relative risk of developing CKD was determined to be 1.86 (95% CI: 1.43–2.41, p < 0.0001) in subjects with MetS.Citation21 However, some authors illustrated that MetS lost its predictive power in late-stage CKD progression in middle age of people or elderly.Citation16,Citation22 There was evidence that it showed a strong link between renal damage and MetS criteria.Citation23 However, above studies did not predict the effect of treating MetS. We discussed that controlled MetS in early-stage CKD appeared to decrease the risk of late-stage CKD, but it seemed that the renal function decline in our selected population (estimated annual GFR decline 7.4 mL/min/1.73 m2 in CMS group and 16.8 mL/min/1.73 m2 in UMS group) conferred faster progression to late-stage CKD as compared to another study (0 mL/min/1.73 m2 in CMS group and 5 mL/min/1.73 m2 UMS group).Citation18 In the consequence of CKD to cardiovascular disease and mortality, it revealed unclear correlation of RKFD, annual eGFR decline >3 mL/min/1.73 m2, in patients from baseline.Citation24,Citation25 Fifty three people (32.7%) at baseline in our study attributed to stage one CKD. High variability and fluctuation in absolute change among those with baseline eGFR > 90 mL/min/1.73 m2 was reported.Citation25 Not all components of MetS in diabetic patients are well controlled. Modification of lifestyle habits involving healthy diet, smoking cessation, physical exercise, and weight loss in obese people may be the most valuable in regarding the progression of early stage of CKD. Recent experience indicates that combining restriction of dietary protein with augmentation of fluid intake decrease CKD progression. Nevertheless, intensive intervention with multi-drug administration and lifestyle modification had sustained beneficial effects on cardiovascular mortality and all cause mortality, and most of the patients reached the therapeutic target of hypertriglyceridemia.Citation26 Based on subgroups B and D in our study, most patients had better control in total cholesterol (decrement of total cholesterol between baseline and follow-up was 10.76 ± 40.76 mg/dL, p = 0.021), but it was difficult to attain the goal of HDL-c (decrement of HDL-c between baseline and follow-up was 3.19 ± 9.92 mg/dL, p = 0.005). Anemia, decreased reserved renal function, hyperuricemia, and serum albumin have been the well-known risk factors for CKD progression. In 2011, Lee et al. demonstrated that only MetS and serum albumin were independent risk factors for CKD progression in early-stage CKD. Using obesity allows for evaluation with the simple metric of body mass index on nutritional assessment in CKD. Each MetS criterion had different predictive power, and fasting glucose was the highest predicting factor for cardiovascular disease among Korean patients with MetS.Citation27 Hypertriglyceridemia was the only independent risk factor for microalbuminuria and the development of late-stage CKD.Citation18 After adjusting multiple variables, Sun et al. reported that central obesity, hypertriglyceridemia, and hypertension had higher risk to develop CKD than hyperglycemia and low HDL-c.Citation28 Subsequently, our study depicted that hypertension had the tendency to predict the development of late-stage CKD. A meta-analysis had also reported that lower mean blood pressure resulted in slower GFR decline in either diabetes or non-diabetes.Citation29
There are several limitations in this study. First, GFR was indirectly measured by a serum creatinine-based MDRD equation, and it was less accurate for subjects with higher GFR than those with lower GFR. Owing to high variability of eGFR, repeated measuring values of eGFR were necessary to reduce intra-individual difference. Second, use of logistic regression analysis required to know the exact time of events. The modeling in the follow-up study used the time when events had already occurred. It may underestimate the association between metabolic components with incident late-stage CKD. Third, patients without regular follow-up were excluded and it could lead to selection bias. Finally, the study population is relatively small and the length of follow-up period is short. Prospective studies with larger sample size and longer length of follow-up duration are needed to confirm our trial.
Conclusions
In summary, our retrospective observational cohort study established a positive and significant correlation of MetS and CKD between CMS and UMS groups. The condition associated with persistence of MetS at baseline and follow-up had higher risk to develop late-stage CKD. The involvement of the metabolic components was positively associated with renal function deterioration. It should be emphasized that hypertension represented greater tendency to renal function deterioration. Treating MetS attenuates CKD progression in patients with early-stage CKD.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
We thank Hsin Yi Cheng, Yu-Tzu Tsao, MD, Jui-Tsung Ting, MD, and Wei-Wen Chang, MD, for the contribution to the data collection and statistic analyses.
References
- Barylski M, Małyszko J, Rysz J, Myśliwiec M, Banach M. Lipids, blood pressure, kidney—What was new in 2011? Arc Med Sci. 2011;7:1055–1066
- Curhan GC. Prediabetes, prehypertension—Is it time for pre-CKD? Clin J Am Soc Nephrol. 2010;5:557–559
- Reaven GM. Insulin resistance and human disease: A short history. J Basic Clin Physiol Pharmacol. 1998;9:387–406
- Liese AD, Mayer-Davis EJ, Haffner SM. Development of the multiple metabolic syndrome: An epidemiologic perspective. Epidemiol Rev. 1998;20:157–172
- Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716
- Tanaka H, Shiohira Y, Uezu Y, Higa A, Iseki K. Metabolic syndrome and chronic kidney disease in Okinawa, Japan. Kidney Int. 2006;69:369–374
- Ritz E. Metabolic syndrome and kidney disease. Blood Purif. 2008;26:59–62
- Luk AO, So WY, Ma RC, et al. Metabolic syndrome predicts new onset of chronic kidney disease in 5,829 patients with type 2 diabetes: A 5-year prospective analysis of the Hong Kong Diabetes Registry. Diabetes Care. 2008;31:2357–2361
- Hwang LC, Bai CH, Chen CJ. Prevalence of obesity and metabolic syndrome in Taiwan. J Formos Med Assoc. 2006;105:626–635
- Nestel P, Lyu R, Low LP, et al. Metabolic syndrome: Recent prevalence in East and Southeast Asian populations. Asia Pac J Clin Nutr. 2007;16:362–367
- NCEP, Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP). JAMA. 2001;285:2486–2497
- Koh KK, Han SH, Quon MJ. Inflammatory markers and the metabolic syndrome: Insights from therapeutic interventions. J Am Coll Cardiol. 2005;46:1978–1985
- Kaysen GA. Hyperlipidemia in chronic kidney disease. Int J Artif Organs 2007;30:987–992
- Alexander MP, Patel TV, Farag YM, Florez A, Rennke HG, Singh AK. Kidney pathological changes in metabolic syndrome: A cross-sectional study. Am J Kidney Dis. 2009;53:751–759
- Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among non-diabetic adults. J Am Soc Nephrol. 2005;16:2134–2140
- Lee CC, Sun CY, Wu IW, Wang SY, Wu MS. Metabolic syndrome loses its predictive power in late-stage chronic kidney disease progression-a paradoxical phenomenon. Clin Nephrol. 2011;75:141–149
- Health Promotion Administration, Ministry of Health and Welfare, Republic of China (Taiwan). Definition of metabolic syndrome 2007 edition. 2007. Available at: http://www.hpa.gov.tw/BHPNet/Web/HealthTopic/TopicArticle.aspx?No=200712250123&parentid=200712250023. Accessed September 20, 2014
- Duran-Perez EG, Almeda-Valdes P, Cuevas-Ramos D, Campos-Barrera E, Muñoz-Hernandez L, Gomez-Perez FJ. Treatment of metabolic syndrome slows progression of diabetic nephropathy. Metab Syndr Relat Disord. 2011;9:483–489
- Tomaszewski M, Charchar F, Maric C, et al. Glomerular hyperfiltration: A new marker of metabolic risk. Kidney Int. 2007;71:816–821
- Hopps E, Noto D, Caimi G, Averna MR. A novel component of the metabolic syndrome: The oxidative stress. Nutr Metab Cardiovasc Dis. 2010;20:72–77
- Tozawa M, Iseki C, Tokashiki K, et al. Metabolic syndrome and risk of developing chronic kidney disease in Japanese adults. Hypertens Res. 2007;30:937–943
- Cheng HT, Huang JW, Chiang CK, Yen CJ, Hung KY, Wu KD. Metabolic syndrome and insulin resistance as risk factors for development of chronic kidney disease and rapid decline in renal function in elderly. J Clin Endocrinol Metab. 2012;97:1268–1276
- Ryu S, Chang Y, Woo HY, et al. Time-dependent association between metabolic syndrome and risk of CKD in Korean men without hypertension or diabetes. Am J Kidney Dis. 2009;53:59–69
- Bauer C, Melamed ML, Hostetter TH. Staging of chronic kidney disease: Time for a course correction. J Am Soc Nephrol. 2008;19:844–846
- Clark WF, Macnab JJ, Sontrop JM, et al. Dipstick proteinuria as a screening strategy to identify rapid renal decline. J Am Soc Nephrol. 2011;22:1729–1736
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591
- Hwang IC, Kim KK, Jee SH, Kang HC. A comparison of predictability of cardiovascular events between each metabolic component in patients with metabolic syndrome based on the revised National Cholesterol Education Program criteria. Yonsei Med J. 2011;52:220–226
- Sun F, Tao Q, Zhan S. Metabolic syndrome and the development of chronic kidney disease among 118924 non-diabetic Taiwanese in a retrospective cohort. Nephrology (Carlton). 2010;15:84–92
- Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: A consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36:646–661