Abstract
Objectives: Acute kidney injury (AKI) is a common complication of cardiac surgery developing in 25–35% cases. Recently, neutrophil gelatinase-associated lipocalin (NGAL) was shown to predict AKI development earlier than serum creatinine. Some studies demonstrated the predictive role of post-operative serum uric acid (SUA) as an early marker of AKI. We aimed to study the role of serum and urine NGAL as well as SUA to predict progression of AKI. Design and methods: This is a prospective observational study of patients undergoing cardiac surgery. Blood and urine samples for measurement of uric acid, serum and urine NGAL levels were collected prior to cardiac surgery (0 h), and in the time course at 2nd and 24th hours after surgery. Patients who developed AKI were divided into two subgroups as progressing and non-progressing AKI. Results: Sixty patients (42 males, 18 females) were included. After cardiac surgery, 40 patients developed AKI, 20 of whom non-progressing AKI, and 20 progressing AKI. All of the markers significantly increased in AKI patients. A receiver operator characteristics (ROC) curve analysis showed higher predictive ability of SUA for progressing AKI compared with serum and urine NGAL. When compared markers obtained at the second hour after surgery, UA had significantly large AUC than NGAL to predict AKI developed at 24 and 48 h, particularly in patients, who require renal replacement therapy (RRT). Conclusion: Uric acid seems to predict the progression of AKI and RRT requirement in patients underwent cardiac surgery better than NGAL.
Introduction
Acute kidney injury (AKI) is considered as an independent risk factor for morbidity and mortality in hospitalized patients, especially in intensive care unit and in patients, undergone major surgery.Citation1,Citation2 The severity and progression of AKI have also deleterious effects on patient outcomes after cardiovascular surgery.Citation3,Citation4 Recently, the definition of AKI was standardized with acute kidney injury network (AKIN)Citation5 criteria.
Several biomarkers have been discovered to predict and diagnose AKI in recent years.Citation6 Neutrophil gelatinase-associated lipocalin (NGAL) is a small protein of the lipocalin superfamily and is expressed by renal tubular cells.Citation7 Both urine and serum NGAL (sNGAL) have been shown to be early predictors of AKI in critically ill patients.Citation8,Citation9 Serum uric acid (SUA) has been found to be a pathogenetic factor in development of AKI. Main mechanisms include renal vasoconstriction, increased inflammation, apoptosis and impaired renal autoregulation,Citation10,Citation11. Recently Ejaz et al.Citation12 showed that SUA level after cardiac surgery was associated with an increased risk for AKI with a comparable power with serum creatinine and novel markers of AKI. However, the authors did not specified exact timing of blood and urine sampling after the completion of cardiac surgery. Since levels of these biomarkers may significantly change within minutes to hours after operation, loosely characterized time-frame may have affected the results of that study. This study did not mention the ability of studied biomarkers to predict progressed patients with AKI either.
Determining which patient will be in a stable phase of AKI (transient elevations of serum creatinine) and which patient will develop consistent and progressive AKI (AKIN stage 2–3) after major surgery is still challenging. Despite the improvements in diagnostic markers, neither of them could predict the progression of AKI nor could determine the requirement of dialysis. Hence, we aimed to examine whether SUA, compared with sNGAL and urine NGAL (uNGAL), could predict the development and progression of AKI in patients with AKI after cardiac surgery with repeated measures of markers in a pre-defined post-operative time frame.
Materials and methods
Study design and patient selection
This is a prospective, observational study of patients who underwent cardiac surgery (elective coronary artery bypass grafting and/or cardiac valve surgery) between July 2011 and February 2012. The study protocol was approved by Local Ethical Committee (No. -217) and informed consent forms were signed by all study participants. The primary endpoint of the study was the development of AKI following open heart surgery. We evaluate the role of serum uric acid in early post-operative prediction of AKI in comparison with serum creatinine, urine and serum NGAL values. AKI was defined as per the KDIGO AKI guideline: increase in serum creatinine by 0.3 mg/dL within 48 h or increase in serum creatinine to 1.5 times baseline.Citation13 All patients who developed AKI were classified as: stage I AKI when serum creatinine increased to 1.5–1.9 times baseline, stage II – increase in serum creatinine to 2.0–2.9 times baseline and stage III – increase in serum creatinine to ≥3.0 times baseline (including initiation of renal replacement therapy).
All patients consecutively admitted to the Department of Cardiovascular Surgery during the aforementioned periods were assessed for eligibility for the study inclusion criteria. Inclusion criteria included age >18 years, elective open heart surgery (irrespective of the specific indication for heart surgery), pre-operative estimated glomerular filtration rate (eGFR) >30 mL/min/1.73 m2 and left ventricle ejection fraction (LVEF) >30%. Patients with urgent and/or emergent cardiac surgery, history of recent acute myocardial infarction within 1 month prior to surgery and suspicion of contrast induced AKI were excluded.
Eighty-five patients were eligible for the study, 85 patients recruited, 10 patients refused and 15 patients excluded from the study due to missing data (missed blood and/or urine collection for lab tests). Finally, 60 patients were included for this study. The laboratory investigators were blinded from data providing clinical outcome of patients until the end of the study. Blood and urine samples were collected independently without taking into account the outcome of patients. All of the markers (serum uric acid, sNGAL, and uNGAL) were measured at the end of the study, when patient recruitment and data collection were stopped.
Medical history, comorbid diseases and current medications were recorded after interview with patients, and/or collected from medical charts. Other variables including cardiac functional parameters, serum creatinine, type of surgery, cardiopulmonary bypass time (CPB), and length of hospital stay were also noted. eGFR was calculated via modification of diet in renal disease (MDRD) equation.Citation14 Blood and urine samples for measurement of serum creatinine, SUA, sNGAL and uNGAL levels were collected prior to cardiac surgery at baseline (0th h), and in the time course at 2nd and 24th hours after the completion of the surgical operation. All of the patients, who were included to the study, were followed-up until they discharge from hospital.
All patients were categorized into AKI and non-AKI groups following cardiac surgery according to aforementioned criteria. Then patients who developed AKI were divided into two subgroups as non-progressing and progressing AKI. Criteria for progression of AKI were as follows: any transition from one stage of AKI to another stage (from stage 1 to 2, from stage 2 to 3) and additional increase in the serum creatinine value for patients with stage 3 AKI or requirement for initiation of renal replacement therapy (RRT).
Biochemical analyses
Coagulated blood samples were collected after 12-h fasting from patients and at frequent intervals after open heart surgery, centrifuged at 3800g for 5 min, and serums were stored at −20 °C until analysis. Spot urine samples were obtained via urinary catheter at baseline and at frequent intervals after initiation of open heart surgery, and centrifuged at 2000g for 5 min, and the supernatants also stored in aliquots at −20 °C. Demographic data, urine and blood samples were collected in a time-frame of 6 months. Serum creatinine and uric acid levels were analyzed on Synchron LX20 system (Beckman Coulter, Brea, CA) with the original Beckman reagents.
Human lipocalin-2/NGAL
Serum and urine NGAL levels were measured by a commercially available kit (Bio Vendor NGAL Elisa kit; Cat no: RD191102200R, BioVendor Research and Diagnostic Products, Brno, Czech Republic). Serum samples were diluted 30× and urine samples were diluted 10× with dilution buffer. Results were expressed as ng/mL.
Surgical protocol
All patients were operated by the same cardiovascular surgery team in the Cardiovascular Department of University Hospital. Anesthesia techniques and surgical procedures (including on-pump and off-pump) were provided as per local protocol of the hospital which were described in detail elsewhere.Citation15
Pre- and post-operation fluid regime
The following fluid protocol was used: patients were fasted for 12 h prior to surgery, and infused by 100 mL/h saline fluid during the 8 h pre-operative period. Patients with DM were infused by 500 mL 10% dextrose + 16 IU insulin + 10 mL 5% potassium chloride. In the post-operative period, patients were infused by 5% 1000 ml dextrose + 16 IU insulin in dose of 100 mL/h.
Statistical analyses
All statistical analyses were performed by using SPSS 17.0 (SPSS Inc., Chicago, IL) statistical package. All data are expressed as mean (SD, standard deviation) for normally and median (IQR, interquartile range) for not normally distributed numerical variables. Categorical variables presented as number (percentage). Non-parametric statistic was performed for data-set analysis. Between-group comparisons were assessed for numerical variables with Kruskal–Wallis and/or Mann–Whitney U-test, and for categorical variables with the Chi-square test or Fisher’s exact test. Univariate logistic regression was used to test the association of several risk factors with development of AKI. Stepwise multivariate logistic regression analysis was used to assess the predictors for AKI development. A receiver operator characteristics curve (ROC curve) analysis was performed to identify the sensitivity and specificity of cut-off values of biomarkers (sNGAL, uNGAL, SUA, and creatinine) in prediction of AKI. Between groups comparison of AUC provided with method developed by DeLong et al.Citation16 A value of р ≤ 0.05 was accepted as statistically significant.
Results
Baseline characteristics
The baseline characteristics of study patients who developed AKI and do not develop AKI (non-AKI) were shown in . There was no difference among the groups in terms of age, gender, underlying chronic diseases except that congestive heart failure was more common in non-progressing and progressing AKI groups compared with the non-AKI group. Baseline pre-operative serum creatinine and eGFR values were significantly lower in patients with AKI compared with non-AKI patients (). Similarly serum NGAL, but not urine NGAL, levels were significantly different between the groups at baseline evaluation. Although the progressing AKI patients have higher pre-operative serum uric acid level compared with other two groups, this did not reach statistically significant level (). Median of ICU length of stay was 8.35 days for patients with progressing AKI and was significantly longer than the other groups. All four deaths occurred in the group of progressing AKI.
Table 1. General characteristics* of the whole study cohort.
AKI patients were classified into two groups according to progression status that defined in the “Materials and methods” section. Out of 60 patients who underwent cardiac surgery, 40 (66.6%) developed AKI. Of these 40 patients, 34 (85% of those patients with AKI) were diagnosed with AKIN stage I and six were diagnosed with AKIN stage II AKI. Of the 34 patients, 19 persisted in stage I, and kidney function recovered following days; five progressed from stage I to stage II, and seven progressed from stage I to stage III. Of the five patients who progressed from stage I to stage II, two progressed from stage II to stage III in the follow-up period. Of the six patients, who diagnosed previously with stage II, one patient recovered kidney function and five patients progressed to stage III AKI. Finally, of 40 patients who developed AKI, 20 patients progressed to a higher degree of AKI (from AKI stage I to stage II–III and/or from stage II to III) during their post-operative follow-up; seven of these 20 progressors received RRT (). Overall four patients died, all of which underwent RRT.
According to the time course levels of serum creatinine, 12 (30%) patients developed AKI within the first 2 h, whereas 21 (52.5%) patients, 5 (12.5%) patients, and 2 (5%) patients were diagnosed with AKI within 12, 24, and 48 h after cardiac surgery, respectively.
Post-operative changes in SUA and serum and urine NGAL
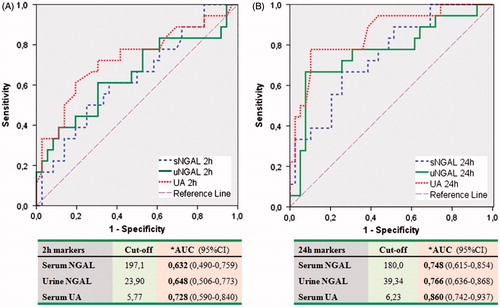
When all three groups were compared with respect to baseline, 2nd and 24th hour values of sNGAL, uNGAL, and SUA levels, only uNGAL and SUA showed consistent increases across the progressing AKI group (). Serum NGAL levels were not different between groups whereas 24th hour sNGAL levels showed a significant increase in patients who developed AKI. ROC curve analysis including all patients who developed AKI showed that the greatest AUC at 2nd and 24th hour post-operative belonged to uNGAL and sNGAL, respectively. On the other hand, when only patients who developed post-operative progressing AKI were taken into consideration, uric acid had the greatest AUCs both for 2nd and 24th hour values ().
Figure 1. Changes in sNGAL, uNGAL, serum creatinine, and uric acid levels after cardiac surgery in non-AKI, non-progressing AKI, and progressing AKI groups.
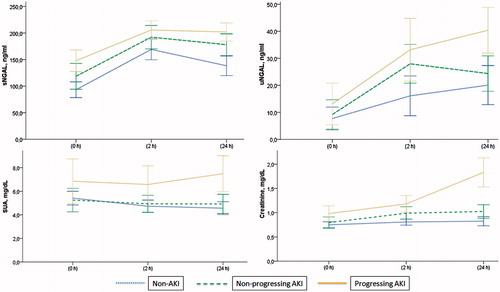
Figure 2. ROC curves showing cut-off points and AUC for 2nd (Panel A) and 24th hour (Panel B) levels of sNGAL, uNGAL, and serum uric acid to predict progressing AKI. Notes: AUC – Area under the ROC curve; ROC – receiver operator characteristics. *Compared by the method of DeLong et al.
We also analyzed whether AKI biomarkers at post-operative 2nd hour would predict AKI at 12th, 24th, and 48th hours post-operative. ROC curve analysis showed that a second hour value of uric acid 5.75 mg/dL and 5.60 mg/dL could predict development of AKI at 48th hour and requirement for renal replacement therapy with a fairly high sensitivity and specificity values ().
Table 2. Second hour markers to predict AKI defined/persisted at 12th, 24th, and 48th hours and requirement of CRRT.
Independent predictors of post-operative AKI development
We performed logistic regression analysis with Backward Stepwise (Conditional) method. When all four AKI biomarkers (serum creatinine, serum uric acid, uNGAL, and sNGAL) were entered into the model, serum uric acid, and urine NGAL emerged as independent predictors of development of progressive AKI ().
Table 3. Multivariate analysis of 2nd hour markers to predict AKI incidence.
Discussion
The salient findings of our study were that increased post-operative SUA levels were associated with the development and progression of AKI better than sNGAL, creatinine, and uNGAL. In addition, both uNGAL and SUA levels, studied after 2 h of completion of open heart surgery, were independent predictors of AKI progression.
Cardiac surgery is an important risk factor for the development of AKI. The incidence of AKI after adult cardiac surgery is about 5–10% and is strongly dependent on pre-existing renal function and the complexity of the surgery.Citation17 Since development of AKI loads heavy burdens both on patients and health system, early detection is of paramount importance. In addition to better appreciation of some clinical risk factors associated with cardiac surgery-associated AKI,Citation18 several biomarkers have been tested for early detection of AKI. The most studied and promising for routine clinical use of these markers is NGAL. Some studies evaluated pre-operative and post-operativeCitation19 biomarkers, while some others evaluated intraoperativeCitation20 temporal relation and predictive ability of several AKI biomarkers. As described in detail in a recent review,Citation21 serum and urine NGAL have been shown in a number of studies as independent predictors of development of post-operative AKI in patients undergoing cardiac surgery. On the other hand, little data are available regarding the role of early post-operative NGAL values in prediction of AKI.
Not all serum creatinine elevations relentlessly progress, rather most increases are mild and reversible and do not confer increased risk for the patient. Therefore, increased serum creatinine values have no high sensitivity and specificity to predict progressive AKI after completion of cardiac surgery. Thus, it is a real clinical need to differentiate which patient developing AKI will progress and which will not.
Koyner et al.Citation4 evaluated whether kidney injury biomarkers measured at the time of first clinical diagnosis of early AKI after cardiac surgery can forecast AKI severity. To the best of our knowledge, this study is the only one apart from ours investigating the predictive role of AKI biomarkers in the progression of AKI once it has developed after cardiac surgery. The authors measured serum and urine NGAL, IL-18, KIM-1, and urine albumin to creatinine ratio (ACR). The highest quintiles of IL-18, ACR, and sNGAL remained associated with AKI progression in a multivariate model corrected for major post-operative AKI risk factors. A notable difference from the current study was that we measured serum and urine NGAL at pre-defined time points irrespective of the development of AKI in all study participants whereas Koyner et al.Citation4 measured biomarkers at the day of post-operative AKI. The authors did not study serum uric acid as a predictive marker in their study either.
Uric acid has been recently implicated in the causation of renal disease, hypertension, and coronary artery disease.Citation22–24 This new paradigm of causation has distinct pathophysiologic pathways different from classical uremic nephropathy, which traditionally described in the setting of tumor lysis syndrome and severe gouty arthritis.Citation25–27 Compelling experimental data and several observational and epidemiologic studies showed a strong association of kidney damage and elevated serum uric acid levels. On the other hand, data are scarce in terms of predictive ability of SUA in AKI. A recent population-based study showed that elevated SUA could predict development of AKI in addition to chronic kidney disease in the very long period.Citation28 Interestingly, the authors found SUA as a significant marker for future development of AKI independent of major confounders, particularly the level of baseline GFR.
Ejaz and coworkersCitation29 evaluated predictive value of SUA in development of AKI in a number of studies. In a small randomized double-blind trial, the authors evaluated effect of reducing SUA levels by rasburicase in patients undergoing cardiac bypass surgery on prevention of AKI development. Although there was no significant difference in post-operative serum creatinine levels between the rasburicase and placebo-treated patients, uNGAL tended to be lower in the rasburicase group. The same group reported a retrospective study in which they found that pre-operative SUA was associated with an increased incidence and a risk for AKI, higher post-operative serum creatinine values, and longer hospital length of stay in patients undergoing cardiac surgery.Citation30 Interestingly this study also showed that lower SUA levels were also associated with an increased risk of developing AKI. Lastly Ejaz et al.Citation12 conducted a prospective observational study in which they evaluated relationships between SUA, uNGAL and interleukin-18, serum monocyte chemoattractant protein-1 and tumor necrosis factor-alpha, and incidence of AKI. The authors collected blood samples within 24 h after initiation of open heart surgery but did not specify a particular time-frame in contrast to our study. They found that third tertile of SUA was associated with 54.5% increased risk for development of AKI. In ROC curve analyses, SUA had larger AUC compared with serum creatinine but comparable AUC compared with aforementioned biomarkers. However, the authors did not classify patients who developed AKI as progressors and non-progressors.
Our findings showed that SUA level was not significantly different between patients at baseline despite a trend toward higher SUA values in patients who would develop progressive AKI later on the course of the post-operative follow-up. However, when we look at the post-operative values, both second and 24th hour post-operative SUA values were significantly higher in patients with progressive AKI compared to patients with non-progressive AKI and patients who did not develop AKI at all. ROC curve analysis revealed that while 2nd hour post-operative uNGAL had the largest AUC for prediction of AKI development at 12th hour, 2nd hour SUA levels had the largest AUC values for prediction of AKI at 24th and 48th hours (, ). Second post-operative hour SUA level had the best sensitivity and specificity to predict future need for RRT in patients undergoing cardiac surgery. We constructed a multivariate model to evaluate independent predictive performances of included biomarkers; 2nd hour serum creatinine value was the single independent predictor of overall development of AKI in all cohort, whereas 2nd hour sNGAL and SUA were independent predictors of progressed AKI cases ().
Some limitations deserve to be mentioned. Our cohort had limited number of patients. We did not collect data regarding medication use including thiazide diuretics, which may affect SUA levels. However, the number of patients with clinically evident heart failure who might have needed diuretics was only six (10%). Diet, fluid therapy, and hydration status of the patients might have affected SUA levels. However, we saw that this issue has not been handled properly in any of the aforementioned studies. Despite above shortcomings, current study evaluated the value of early post-operative SUA level in prediction of risk of progressive AKI in a well characterized study design.
In conclusion, this study showed that early post-operative SUA could distinguish cardiac surgery patients who would later develop progressive AKI. Second hour post-operative SUA had a fairly high sensitivity and specificity in ROC analysis and was also appeared as an independent predictor of progressive AKI development.
Acknowledgments
Dr A.G. was supported in performing the present study by Long-Term Fellowship training award from the European Renal Association – European Dialysis and Transplant Association (ERA-EDTA LTF 65-2010). This study was awarded first best oral presentation at 29th annual congress of Turkish Society of Nephrology (2012).
Declaration of interest
The authors report no conflicts of interest in this work.
References
- Liano F, Junco E, Pascual J, Madero R, Verde E. The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. The Madrid Acute Renal Failure Study Group. Kidney Int Suppl. 1998;66:S16–S24
- Hilberman M, Myers BD, Carrie BJ, Derby G, Jamison RL, Stinson EB. Acute renal failure following cardiac surgery. J Thorac Cardiovasc Surg. 1979;77(6):880–888
- Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104(4):343–348
- Koyner JL, Garg AX, Coca SG, et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol. 2012;23(5):905–914
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31
- Akcay A, Turkmen K, Lee D, Edelstein CL. Update on the diagnosis and management of acute kidney injury. Int J Nephrol Renovasc Dis. 2010;3:129–140
- Schmidt-Ott KM, Mori K, Li JY, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18(2):407–413
- Siew ED, Ware LB, Gebretsadik T, et al. Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol. 2009;20(8):1823–1832
- Cruz DN, de Cal M, Garzotto F, et al. Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med. 2010;36(3):444–451
- Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16(12):3553–3562
- Sanchez-Lozada LG, Tapia E, Santamaria J, et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67(1):237–247
- Ejaz AA, Kambhampati G, Ejaz NI, et al. Post-operative serum uric acid and acute kidney injury. J Nephrol. 2012;25(4):497–505
- KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney International Supplements. 2012;2(1):8–12
- The Modification of Diet in Renal Disease Study: design, methods, and results from the feasibility study. Am J Kidney Dis. 1992;20(1):18–33
- Celik JB, Gormus N, Topal A, Okesli S, Solak H. Effect of off-pump and on-pump coronary artery bypass grafting on renal function. Ren Fail. 2005;27(2):183–188
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44(3):837–845
- Bellomo R, Auriemma S, Fabbri A, et al. The pathophysiology of cardiac surgery-associated acute kidney injury (CSA-AKI). Int J Artif Organs. 2008;31(2):166–178
- Shaw A. Update on acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg. 2012;143(3):676–681
- Liebetrau C, Dorr O, Baumgarten H, et al. Neutrophil gelatinase-associated lipocalin (NGAL) for the early detection of cardiac surgery associated acute kidney injury. Scand J Clin Lab Invest. 2013;73:392–399
- Krawczeski CD, Goldstein SL, Woo JG, et al. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol. 2011;58(22):2301–2309
- Vanmassenhove J, Vanholder R, Nagler E, Van Biesen W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: An in-depth review of the literature. Nephrol Dial Transplant. 2013;28(2):254–273
- Kanbay M, Segal M, Afsar B, Kang DH, Rodriguez-Iturbe B, Johnson RJ. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart. 2013;99(11):759–766
- Kanbay M, Yilmaz MI, Sonmez A, et al. Serum uric acid independently predicts cardiovascular events in advanced nephropathy. Am J Nephrol. 2012;36(4):324–331
- Turgut F, Kasapoglu B, Kanbay M. Uric acid, cardiovascular mortality, and long-term outcomes in CKD. Am J Kidney Dis. 2009;54(3):582; author reply 582–583
- Ejaz AA, Mu W, Kang DH, et al. Could uric acid have a role in acute renal failure? Clin J Am Soc Nephrol. 2007;2(1):16–21
- Ejaz AA, Beaver TM, Shimada M, et al. Uric acid: A novel risk factor for acute kidney injury in high-risk cardiac surgery patients? Am J Nephrol. 2009;30(5):425–429
- Shimada M, Dass B, Ejaz AA. Paradigm shift in the role of uric acid in acute kidney injury. Semin Nephrol. 2011;31(5):453–458
- Ben-Dov IZ, Kark JD. Serum uric acid is a GFR-independent long-term predictor of acute and chronic renal insufficiency: The Jerusalem Lipid Research Clinic cohort study. Nephrol Dial Transplant. 2011;26(8):2558–2566
- Ejaz AA, Dass B, Lingegowda V, et al. Effect of uric acid lowering therapy on the prevention of acute kidney injury in cardiovascular surgery. Int Urol Nephrol. 2013;45(2):449–458
- Lapsia V, Johnson RJ, Dass B, et al. Elevated uric acid increases the risk for acute kidney injury. Am J Med. 2012;125(3):302 e9–17
