Abstract
The combination of hemodialysis–hemoperfusion (HDHP) has been proved to be superior to hemodialysis (HD) in eliminating uremic toxins. There are two methods of combination of HD and HP: the HP regime is utilized during the first two-hour an early HP conducted HDHP (EHDHP) or the last two-hour late HP conducted HDHP (LHDHP) of 4 h regular HD session. The present study was to compare these two methods in uremic toxins removal. Twenty adult chronic HD patients were enrolled in this self-control method study. The patients were randomized to receive one session of EHDHP or LHDHP. Two weeks later, the dialysis modalities were switched. The reduction ratio (RR) of targeted uremic toxins for each session was assessed. Both EHDHP and LHDHP showed a significant removal of small water-soluble solutes, middle-sized toxins and cytokines as well (p < 0.05). There were no significant differences between two methods in RR of small water-soluble solutes, like urea and creatinine. For middle-sized molecules and cytokines, such as PTH, β2-M, IL-1, IL-6, and TNF-α, the RR was markedly increased in LHDHP than that in EHDHP (p < 0.05). LHDHP showed no more intradialytic events than EHDHP. The combination of HD and HP in the last two hours in one hemodialysis session had more effect on eliminating middle-sized toxins and cytokines.
Introduction
It has been proved by various clinical studies that parathyroid hormone (PTH) accumulation results in renal osteodystrophy and ectopic calcification,Citation1,Citation2 the accumulation of beta microglobulin (β2-M) leads to amyloid change and carpal tunnel syndrome,Citation3,Citation4 and the accumulation of cytokines, such as interleukin-1 (IL-1), interleukin-6 (IL-6) and tumor necrosis factor (TNF-α) result in chronic systemic inflammation.Citation5–8 Many of these complications are irreversible by standard and conventional hemodialysis (HD) and pose a challenge to the long-term survival of patients on dialysis.Citation9
Various strategies have been developed to increase the clearance of toxins above including on-line hemofiltrationCitation10 and hemodiafiltration.Citation11 An alternative approach, comprising of sequential hemodialysis and hemoperfusion (HDHP), has been used for about 50 years and proved to be excel over HD in regular elimination of middle-sized molecules and cytokines.Citation12–15
In clinical use, HDHP is usually conducted by adding a commercially available hemoperfusion (HP) apparatus into the dialysis circuit in series with a standard hemodialyzer. The HP apparatus generally contains 80–300 g of activated charcoal-coated particles effective for continuous use of approximate two hours. The HP apparatus can be connected with the dialysis circuit either during the first two hours of the single dialysis session, i.e. an early HP conducted HDHP (EHDHP), or during the last two hours of the single dialysis session, i.e. a late HP conducted HDHP (LHDHP). As the insertion of a HP apparatus into the dialysis circuit during the late course of HD may provoke the status of hypovolemia, EHDHP has been the most widely used mode in most of the dialysis centers,Citation15,Citation16 to avoid the ultrafiltration-related interdialysis events, such as interdialysis hypotension (IHD).
There are little differences between EHDHP and LHDHP in terms of treatment duration, consumables, and cost. However, whether the timing of HP component in a single HDHP treatment would affect the efficacy of uremic toxins removal is far less investigated, and few studies have conducted so far to compare EHDHP and LHDHP side by side. The present study was designed to compare the effect of EHDHP and LHDHP on uremic toxins removal in self-controlled patients, and to determine the optimal timing of a HP component in combined HDHP treatment.
Materials and methods
Patients
A total of 20 adult patients (mean age 51.3 ± 17.9 years, 10 males and 10 females) on chronic HD from the hemodialysis department at Shanghai Tongji Hospital were enrolled in the study between July 2012 and January 2013. These patients were on a maintenance dialysis of three times per week for at least 6 months. Their mean dialysis duration was 48.4 ± 9.3 months. The dry weight and eK/tV were 62.7 ± 11.6 kg and 1.4 ± 0.2, respectively. The primary renal diseases were glomerulonephritis (n = 7), diabetic nephropathy (n = 5), hypertension (n = 5), polycystic kidney (n = 1), obstructive nephropathy (n = 1), and interstitial nephritis (n = 1). Patients with tumors, acute or chronic infection, and active autoimmune diseases were excluded. Patients who had taken glucocorticoids and immunosuppressants in the past three months or who were suffering from heart failure or pulmonary edema were also excluded.
Study design
The study was a single center, prospective, and randomized cross-over comparison of EHDHP and LHDHP. The local ethics committee approved the study protocol and informed consent was obtained from all subjects. After inclusion, 20 patients were randomized to receive on session of EHDHP or LHDHP. Thereafter, the dialysis modalities were switched. The time interval between the two successive treatments of HDHP was two weeks.
Systems and treatment strategies
All treatments were performed with Dialog Advanced, B. Braun dialysis machines (Kronberg, Germany). HD and HP were conducted using polysulfone dialyzers (Rexeed 13L; Asahi Kasei corporation, Tokyo, Japan) and polyvinyl alcohol 60 (PVA-60) activated charcoal-coated apparatus (YTS-100; Zibo Kang Bei Group, Biological Material Co. Ltd., Zibo, Shandong, China), respectively. Two schemes were used for HDHP. In EHDHP, HP apparatus was added into the HD circuit in the first two hours where the blood flow rate was maintained between 150 and 200 mL/min, followed by HD alone for the next two hours with the blood flow rate 200–250 mL/min. Similarly, the HP apparatus was connected into the HD circuit during the last two hours instead of the first two hours in a LHDHP.
Blood pressure measurement
Blood pressure (BP) measurement was standardized. Only one type of automatic BP equipment was used (Gambro BP 100, Stockholm, Sweden). BP was measured in the supine position 5 min before the session, every 60 min during the session, at the end of the session just before extracorporeal volume infusion and if symptoms of hypotension occurred.
Intradialytic events
Intradialytic events were classified as symptomatic hypotension, cramps, chest pain. Symptomatic hypotension was defined as a reduction of the systolic blood pressure below 100 mmHg associated with reactions of the patient prompting nurse intervention, such as placing the patient in Trendelenburg’s position, reducing the ultrafiltration rate, or infusing intravenous fluids. An asymptomatic fall of blood pressure is not believed to be of clinical relevance and therefore was not evaluated.
Sample collection and analysis
Sampling was performed according to the National Kidney Foundation Disease Outcomes Quality Initiative Guidelines.Citation17 Blood samples were collected before and after a single HDHP treatment and stored at −70 °C until analysis. All routine serum analyses, as well as PTH and β2-M, were carried out in the biochemistry laboratory in the hospital. The serum levels of IL-1, IL-6, and TNF-α were analyzed using commercially available ELISA kits from Shanghai Bogoo Biotechnology. Co. Ltd, Shanghai, China.
Molecular size and correction of solutes concentrations because of ultrafiltration
According to the criteria proposed by the European Uremic Toxin Work Group, solutes with molecular weight (MW) ranging from 500 Da to 60 kDa were defined as middle-sized molecules, which are not at all or less efficiently dialyzable.Citation18 Molecules smaller than 500 Da were defined as small molecules. Serum concentrations of PTH, β2-M, IL-1, IL-6, and TNF-α after HDHP (Cpost(c)) were corrected for the extracellular volume changes based on differences in the patients’ pre-HDHP body weight (BWpre) and post-HDHP body weight (BWpost): Cpost/corr = Cpost/[1 + (BWpre − BWpost)/0.2BWpost]. Reduction ratio (RR) was calculated according to the formula of RR (%) = [(C0 − Cpost)/C0] × 100, where C was for concentration either before (C0) or after treatment (Cpost).Citation19
Statistical analysis
All continuous variables were tested for normal distribution with the Kolmogorov–Smirnov test before further statistical analysis. Quantitative data were expressed as means ± SD if not otherwise indicated. Qualitative (Categorical) data were presented as percentages. The values of all the parameters at the start of each HDHP mode and RR of each HDHP mode were compared by means of Student’s t-test for unpaired data. The comparisons of uremic toxins concentrations between pre-HDHP and post-HDHP were performed using paired-sample Student’s t-test. test was used to compare the frequency of pooled symptoms for intradialytic events associated with the two schemes of HDHP. Differences were considered significant when p values were less than 0.05. Medcalc Statistical Package (version 8.0, bvba, Ostend, Belgium) was used to analyze data.
Results
The treatment characteristics
As listed in , there are no significant differences in blood flow, treatment duration, pre-HDHP body weight, and weight loss between the two modes.
Table 1. Treatment characteristics of patients undergoing two experimental HDHP.
Comparison of EHDHP versus LHDHP in small water-soluble solutes removal
The serum concentrations of urea and creatinine at the start of EHDHP and LHDHP were similar (). Both treatment modes resulted in a significant decrease of the serum concentrations of urea and creatinine (). The urea and creatinine RR were 71.1 ± 6.1% and 63.4 ± 11.6%, respectively, in EHDHP, and 72.2 ± 10.0% and 68.1 ± 13.7% in LHDHP, with the differences between the two modes not being significant (). In summary, there were no differences in small water-soluble solutes removal between EHDHP and LHDHP.
Figure 1. The comparison of the RR for small water-soluble solutes between two HDHP modes. Data are depicted as % (means ± SD). There were no significant differences in RR of urea and Cr between two methods. Cr, creatinine.
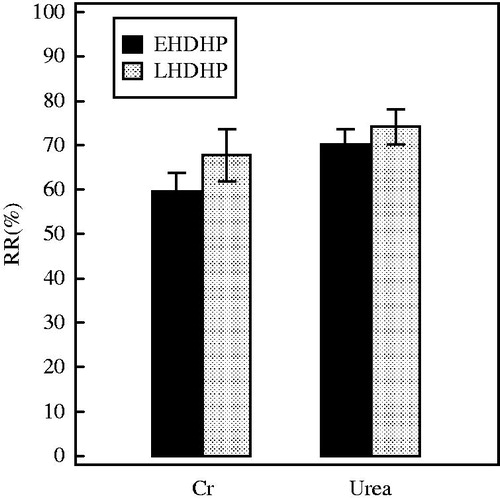
Table 2. Uremic toxins concentrations in pre-HDHP and post-HDHP specimens.
Comparison of EHDHP versus LHDHP in middle-sized molecules removal
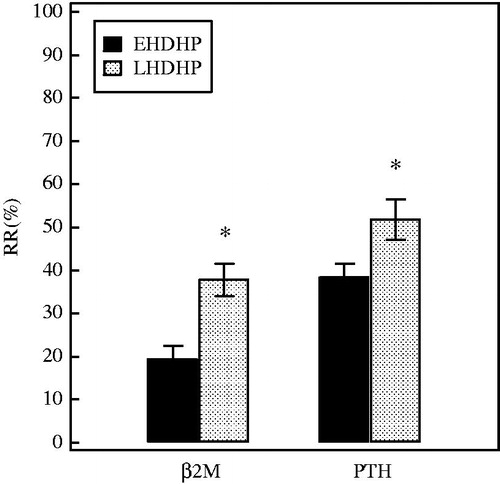
The middle-sized molecules had a similar start concentration (). Both EHDHP and LHDHP led to a significant reduction of the serum concentrations of PTH and β2-M (). The RRs of PTH and β2-M in LHDHP (54.4 ± 14.2 and 38.0 ± 9.3, respectively) were greater than that in EHDHP (40.3 ± 5.4 and 18.1 ± 4.8) (p = 0.0068 and <0.0001, respectively) (). In summary, LHDHP provides significantly more removal of middle-sized molecules than EHDHP.
Figure 2. The comparison of the RR for middle-sized molecules between two HDHP modes. The concentrations of middle-sized molecules were corrected for hemoconcentration because of ultrafiltration, see “Materials and methods” section. Data are depicted as % (means ± SD). *p < 0.05 versus EHDHP. PTH, parathyroid hormone; β2-M, Beta 2 microglobulin.
Comparison of EHDHP versus LHDHP in cytokines removal
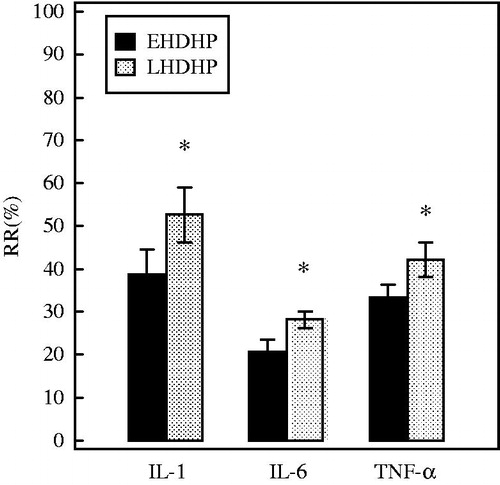
The start values of the cytokines () were similar. Serum levels of the cytokines were significantly decreased by both treatment modes (). The RRs of IL-1, IL-6, and TNF-α were higher in LHDHP (48.9± 15.3, 27.6 ± 4.5, and 43.1 ± 7.5 respectively) than that in EHDHP (37.7 ± 11.8, 20.9 ± 5.9 and 34.2 ± 7.0) (p = 0.0027, 0.0001, and 0.0002, respectively) (). In summary, LHDHP is superior to EHDHP in targeted cytokines removal.
Figure 3. The comparison of the RR for cytokines between two HDHP modes. The concentrations of cytokines were corrected for hemoconcentration because of ultrafiltration, see “Materials and methods” section. Data are depicted as % (means ± SD). *p < 0.05 versus EHDHP. IL-1, interleukin-1; IL-6, interleukin-6; TNF-α, tumor necrosis factor.
Intradialytic events
Within 40 HDHP treatment sessions, 22 intradialytic events were observed. Hypotension and symptoms occurred in 45% of EHDHP, compared with 65% of LHDHP treatment (p = 0.341). Hypotension was the most commonly observed event, but was not statistically different between the groups ().
Table 3. Interdialytic events during HDHP treatments.
Discussion
In our opinion, and from research conducted amongst reputed medical database, this is the first study that compared the timing of HP component in single HDHP treatment, with respect to uremic toxins removal. Three major findings could be established in the present study: (1) The combined therapy of HP with HD led to a remarkable removal of uremic toxins, not only for small water-soluble solutes, but also for middle-sized molecules and cytokines as well; (2) LHDHP excelled over EHDHP in the removal of middle-sized molecules and cytokines, in the context of the improved RRs for the full range of molecules investigated; and (3) LHDHP did not result in additional adverse effects, such as symptomatic hypotension, when compared with EHDHP.
Activated charcoal, which was used in our study, is a kind of sorbents that adsorb molecules by Van der wall’s forces, electrostatic attraction, and/or hydrophobic affinity. Maximal adsorptive capacity is achieved by inducing a high surface porosity and large surface area (approximately 1000 m2/g). Previous studies have observed an unspecific adsorption of charcoal to creatinine and uric acid.Citation20,Citation21 As a result, a reduction of charcoal’s actual surface area for middle-sized molecules can be assumed. Theoretically, the higher the concentration of small water-soluble solutes maintains in blood, the more the charcoal’s surface area will be occupied. If so, a 2-hour diffusive procedure (HD) prior to HP may lead to a considerable clearance of small water-soluble solutes and further increase charcoal’s actual surface area for middle-sized molecules.
The internal burden of molecules around the charcoal is another factor that affects the efficacy of HP.Citation22 The heavier the internal burden of molecules maintains around the charcoal, the more easily the charcoal will get fully saturated. For a PVA-60-coated HP apparatus, the internal burden of molecules is further determined by their plasma concentration and their coating diffusion rate.Citation20 The removal of plasma water during ultrafiltration usually leads to a decrease in plasma volume and a concomitant increase in the concentration of middle-sized molecules not removed by low-flux HD.Citation23,Citation24 In such a case, a HP apparatus which introduced during the late course of HD may suffer from a relatively higher plasma concentrations of middle-sized molecules as well as cytokines, thus acquiring a more accelerated coating diffusion rate (due to the increased concentration gradient of molecules across the coating) and get fully saturated, in comparison with that introduced during the early course of HD.
In theory, the addition of a HP apparatus during the late course of HD may cause more adverse events in association with the increasing loss of extracellular fluid. These included the aggravation of dialysis-related hypotension and other intradialytic events. However, we did not encounter these problems in this study. This could possibly be due to the low blood volume priming of the HP apparatus utilized in our study.
Conclusions
A charcoal-based HP component, which was added during the late course of the regular HD session, is more likely for an enhanced elimination of middle-sized molecules and cytokines without significant intradialytic events. Therefore, it should be recommended as the first choice in clinical practice.
Limitations
There are several issues that should be further addressed. Firstly, an intra-dialysis elevation of middle-sized molecules and cytokines in blood, induced by ultrafiltration, was assumed to be responsible for the improved performance of LHDHP. However, we failed to detect the interdialysis concentrations of targeted molecules of middle-size. Secondly, as we used a charcoal-based HP apparatus in this study and charcoal’s adsorption properties may be different from resin, the results of any current study cannot be directly generalized to the resin-based HDHP. Finally, the number of patients was relatively small. A larger study population and a longitudinal study design, including more HDHP sessions, are needed to observe the long-term benefit of LHDHP in patients with ESRD.
Acknowledgments
The authors are grateful to all the staff in Blood Purification Center, Tongji Hospital, Tongji University.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hörl WH. The clinical consequences of secondary hyperparathyroidism: Focus on clinical outcomes. Nephrol Dial Transplant. 2004;19(Suppl 5):2–8
- De Francisco AM, Cassidy MJ, Owen JP, et al. Ectopic calcification. The role of parathyroid hormone. Proc Eur Dial Transplant Assoc Eur Ren Assoc. 1985;21:888–894
- Dember LM, Jaber BL. Dialysis-related amyloidosis: Late finding or hidden epidemic? Semin Dial. 2006;19(2):105–109
- Schiffl H. Carpal tunnel syndrome in patients on intermittent hemodialysis. Blood Purif. 2012;34(3–4):332
- Herbelin A, Nguyen AT, Zingraff J, et al. Influence of uremia and hemodialysis on circulating interleukin-1 and tumor necrosis factor alpha. Kidney Int. 1990;37(1):116–125
- Vanholder R, De Smet R, Glorieux G, et al. Review on uremic toxins classification concentration, and interindividual variability. Kidney Int. 2003;63(5):1934–1943
- Schömig M, Eisenhardt A, Ritz E. The microinflammatory state of uremia. Blood Purif. 2000;18(4):327–332
- Zeceali C, Tripepi G, Mallamaci F. Dissecting inflammation in ESRD: Do cytokines and C-reactive protein have a complementary prognostic value for mortality in patient. Am Soc Nephol. 2006;17(12 Suppl 3):169–173
- USRDS. Excerpts from the United States renal data system 1998 annual data report. Patient mortality and survival. Am J Kidney Dis. 1998;32(2 Suppl 1):69–80
- Henderson LW, Colton CK, Ford CA, et al. Kinetics of hemodiafiltration. II. Clinical characterization of a new blood cleansing modality. J Lab Clin Med. 1975;85(3):372–391
- Leber HW, Wizemann V, Goubeaud G, et al. Hemodiafiltration: A new alternative to hemofiltration and conventional hemodialysis. Artif Organs. 1978;2(2):150–153
- Trznadel K, walasek L, Kidawa Z, et al. Comparative studies on the effect of hemoperfusion and hemodialysis on the elimination of some uremic toxins. Clin Nephrol. 1978;10:229–232
- Bonomini V, Stefoni S, Casciani CU, et al. Multicentric experience with combined hemodialysis/hemoperfusion in chronic uremia. Contrib Nephol. 1982;29:133–142
- Stefoni S, Coli L, Feliciangli G, et al. Regular hemoperfusion in regular dialysis treatment. A long-term study. Int J Artif Organs. 1980;3(6):348–353
- Chen SJ, Jiang GR, Shan JP, et al. Combination of maintenance hemodialysis with hemoperfusion: A safe and effective model of artificial kidney. Int J Artif Organs. 2011;34(4):339–347
- Li-Ying Miao, Bin Zhu, Xiao-Zhou He, et al. Effects of three blood purification methods on serum fibroblast growth factor-23 clearance in patients with hyperphosphatemia undergoing maintenance hemodialysis. Exp Ther Med. 2014;7(4):947–952
- NKFI. K/DOQI Clinical Practice Guidelines for Hemodialysis Adequacy: Update 2000. Am J Kidney Dis. 2001;37(1 Suppl 1):S7–S64
- Vanholder R, De Smet R, Glorieux G, et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–1943
- Bergstrom J, Wehle B. No change in corrected beta2-microgobulin concentration after cuprophane hemodialysis. Lancet. 1987;1:628–629
- Winchester JF, Ratcliffe JG, Carlyle E, et al. Solute, amino acid, and hormone changes with coated charcoal hemoperfusion in uremia. Kidney Int. 1978;14(1):74–81
- Bonomini V, Stefoni S, Feliciangeli G, et al. Shortened treatment time by combined hemodialysis and hemoperfusion. Contr Nephrol. 1985;44:57–64
- Winchester JF. Sorbent hemoperfusion in end-stage renal disease: An in-depth review. Adv Renal Replace Ther. 2002;9(1):19–25
- Chnediatz D, Putz-Bankuti C, Ribitsch W, et al. Correction of plasma concentrations for effects of hemoconcentration or hemodilution. ASAIO J. 2012;58(2):160–162
- Tae Yamamoto, Marcelo M. Nascimento, Shirley Y. Hayashi, et al. Changes in circulating biomarkers during a single hemodialysis session. Hemodialysis Intern. 2013;17:59–66

