Abstract
Aim: To assess the effects and safety of iron-based phosphate binders in adult patients receiving dialysis. Methods: We electronically searched MEDLINE, EMBASE, CENTRAL, and CBM for randomized controlled trials about iron-based phosphate binders in adult dialysis patients. Study quality was assessed using the criteria outlined in the Cochrane Handbook for Systematic Reviews of intervention. Meta-analysis was conducted by RevMan 5.3. Results: Eight studies with 2018 participants were eligible for our meta-analysis. Iron-based phosphate binders were superior to placebo (MD = −2.43 mg/dL, 95% CI: −3.18 to −1.68, p < 0.00001) and as efficient as sevelamer (MD = 0.04 mg/dL, 95% CI: −0.29 to 0.36, p = 0.83) in reducing serum phosphorus in dialysis patients. No significant differences were found in all adverse events (OR = 1.30, 95% CI: 0.77 to 2.20, p = 0.32) between iron-based phosphate binders and placebo. Iron-based phosphate binders were associated with significant higher serum iron (MD = 9.39 ng/mL, 95% CI 1.48 to 17.30, p = 0.02), higher serum transferring saturation (MD = 6.29%, 95% CI 2.72 to 9.87, p = 0.0006) and lower serum total iron binding capacity (MD = −23.13 µg/dL, 95% CI −35.69 to −10.58, p = 0.0003) in comparison to placebo. Conclusion: Iron-based phosphate binders are as effective as sevelamer and well tolerated for hyperphosphatemia in dialysis patients. Iron-based phosphate binders appear to have a beneficial effect on renal anemia in patients receiving dialysis. Therefore, iron-based phosphate binders may represent a new treatment option for dialysis patients.
Introduction
Dialysis, a process for removing waste and excess water from the blood, is used as an artificial replacement for lost kidney function in people with renal failure. With average survival of 3–5 years in the USA, prevalence of dialysis is nearing 1800 cases per million.Citation1 Medicare expenditures were US$ 87 945 for hemodialysis, $71 630 for peritoneal dialysis per person per year in the USA in 2011.Citation2 Hyperphosphatemia is a universal complication in dialysis patients. Clinical evidence demonstrates that hyperphosphatemia is associated with an adverse effect on renal or cardiovascular function and an increased mortality risk, independent of other traditional risk factors.Citation3–5 The Kidney Disease Outcomes Quality Initiative (KDOQI) in the USA have recommended serum phosphorus targets of 3.5–5.5 mg/dL (1.13–1.78 mmol/L) in patients receiving dialysis.Citation6 Despite dietary restriction and adequate dialysis, the majority of dialysis patients suffering from hyperphosphatemia still need oral phosphate binders to control their phosphate levels and thereby reduce mortality.
Calcium-based phosphate binders, while moderately effective and inexpensive, have been used as the first-line therapy. But these agents are associated with hypercalcemia, soft tissue and cardiovascular calcification,Citation7 suppression of parathyroid gland and adynamic bone disease. Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend restricting the use of calcium-based phosphate binders in patients with persistent or recurrent hypercalcemia or arterial calcification, or both.Citation8 Sevelamer, a calcium- and metal-free synthetic phosphate-binding agent, is a cationic polymer that binds phosphate anions through ion exchange and hydrogen binding in the duodenum. But it is associated with metabolic acidosis,Citation9 gastrointestinal disorders, and high pill burden. Recently, numerous kinds of iron salts that have phosphorus-binding properties have been studied in animal models and human trials. Ferric citrate is designated as a “Generally Recognized as Safe” chemical by the USA Food and Drug Administration, and has been used as a food additive. Ferric citrate hydrate (JTT-751) is a novel phosphate binder containing ferric citrate as an active ingredient.Citation10,Citation11 JTT-751 has a larger surface area and faster dissolution rate than ferric citrate, and is expected to demonstrate efficacy as a phosphate binder.Citation12 SBR759, an iron (III) oxide-hydroxide (FeO(OH))–starch–sucrose–carbonate complex,Citation13 binds to phosphates in food via ligand exchange mechanism in the gastrointestinal tract. Sucroferric oxyhydroxide (PA21) is a new calcium-free polynuclear iron(III)-oxyhydroxide phosphate binder with a high phosphate binding capacity over a wide pH range.Citation14 Fermagate (iron–magnesium hydroxycarbonate) contains magnesium and ferric iron held in an insoluble tight crystalline-layered structure, with carbonate groups, which are exchanged for phosphate, lying between the layers. Differences between phosphate binders exist with respect to the clinical efficacy, possible side-effect profiles, as well as market prices. The optimal use of iron-based phosphate binders requires consideration. We conducted this review to assess the effects and safety of iron-based phosphate binders in serum biochemical endpoints and adverse events in dialysis patients.
Subjects and methods
Inclusion criteria
Our review included randomized controlled trials about iron-based phosphate binders in adult patients (age ≥ 18 years) receiving dialysis. There were no language limitations. Outcomes include: serum phosphorus (mg/dL), serum calcium (mg/dL), serum calcium × phosphorus product (mg2/dL2), serum intact parathyroid hormone (iPTH) (pg/mL), serum hemoglobin (g/dL), iron-related parameters, such as serum iron (µg/dL), serum ferritin (ng/mL), serum transferring saturation (TSAT) (%), and serum total iron binding capacity (TIBC) (µg/dL), adverse events and all-cause mortality.
Search strategy
Two authors electronically searched MEDLINE, EMBASE, Cochrane Controlled Trial Register of Controlled Trials (CENTRAL), and Chinese Biological Medical Database (CBM), the search was updated to 30 April 2014. The structured search strategies used the following format of search terms: (“iron” or “ferric” or “iron-based” or “JTT-751” or “PA21” or “SBR759” or “iron-containing”) and (“phosphorus” or “phosphate” or “hyperphosphatemia”). Search was limited to human subjects and randomized controlled trials. No language restrictions were applied. In addition, the reference lists of identified studies were checked manually to find other potentially eligible studies, this process was performed iteratively until no additional trials could be identified. We also searched World Health Organization International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/) and Clinical Trial Registries (http://clinicaltrials.gov/) for relevant published or ongoing clinical trials. We sought for other unpublished trials through electronic searches or discussions with other experts in this field.
Selection of studies
The titles and abstracts obtained by the search strategy were screened independently by two authors, who discarded studies that were not applicable. Studies and reviews that might have included relevant data or information were retained initially. If necessary, the full text of these studies was reviewed to determine which studies satisfied the inclusion criteria. Differences regarding study selection were resolved by discussion with a third review author.
Data extraction
Studies published in non-English language journals were translated into English before assessment. When more than one publication of one study existed, reports were grouped together or the publication with the most complete data was used in the analyses. Data from eligible studies were extracted by two authors independently on the characteristics of methods (study design, time frame, treatment duration, lost to follow-up), participants (setting, age, sex, country, and exclusion criteria), interventions (drug name, dosage, frequency, mode of administration, duration, and co-interventions) and outcomes. If data was not available in the published reports or there was no detail description of study method, the original authors were contacted by us via email. Discrepancies between the assessments of the two data extractors were resolved by discussion with an arbitrator.
Quality assessment
Two authors who were not blind to the authorship or journal of publication independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of intervention. Discrepancies were resolved by discussion with a third author. The quality items assessed were random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting and other bias. Each domain was classified as having “low risk of bias”, “high risk of bias” or “unclear risk of bias”.
Data analysis
Dichotomous data were analyzed using odds ratio (OR) with its 95% confidence interval (CI). For continuous data, the mean differences (MDs) or the standard mean differences (SMDs) with their 95% CI were computed. Heterogeneity across included studies was tested by using the heterogeneity Chi2 and I2 statistics, which is a quantitative measure of inconsistency across studies. Studies with an I2 statistic of 25 to 50% were considered to have low heterogeneity, those with an I2 statistic of 50 to 75% were considered to have moderate heterogeneity, and those with an I2 statistic of greater than 75% were considered to have a high degree of heterogeneity. Studies with the I2 > 50% or p < 0.10 were identified substantial heterogeneity. If there was no significant heterogeneity, the fixed-effects model was used. In contrast, the random-effects model was used in cases of significant heterogeneity. Potential causes of heterogeneity were explored by carrying out sensitivity and subgroup analyses if available. Sensitivity analyses were performed by switching between the fixed-effects and random-effects model to assess the stability of the results. Owing to the limited number (below 10) of studies included in each analysis, publication bias was not assessed. All statistical analyses of included studies were performed using Review Manager, Version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). A p value of less than 0.05 was considered statistically significant.
Results
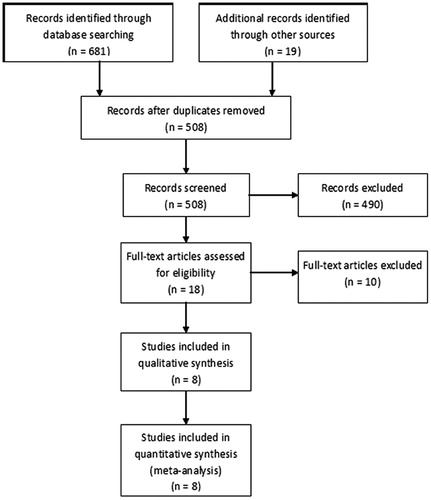
A total of 700 citations were identified by the initial combined search (126 through MEDLINE, 395 through EMBASE, 157 through CENTRAL, 3 through CBM, and 19 through other sources). A total of 192 studies were discarded because of duplicate studies, and 490 studies were excluded based on the titles and abstracts due to irrelevant outcomes, irrelevant participants, not randomized controlled trials, or ongoing trials without available data. The remaining 18 full-text articles were reviewed for more detailed assessment, and 10 studies were excluded because of duplicate reports. Finally, 8 studiesCitation15–22 with 2018 participants met our inclusion criteria and were included in our meta-analysis. The selection process for studies included in the systematic review is shown in . No author responded to our requests for additional data or clarification of study methods.
The main characteristics of the included studies are summarized in . These studies were published in English from 2002 to 2014. Treatment duration ranged from 3 to 24 weeks and sample size varied from 54 to 1059. The mean age of the participants ranged from 52.5 to 62.8 years. Seven studiesCitation15–19,Citation21,Citation22 were parallel randomized controlled trials, while one studyCitation20 was cross-over randomized controlled trial. Three studiesCitation17,Citation18,Citation22 compared iron-based phosphate binders with placebo, four studiesCitation16,Citation17,Citation19,Citation21 made comparison between iron-based phosphate binders and sevelamer, and one studyCitation20 compared ferric citrate with calcium carbonate. Of the eight included trials, fiveCitation15,Citation17,Citation20–22 were conducted in Asia. Two studiesCitation16,Citation17 recruited both hemodialysis and peritoneal dialysis patients and six studiesCitation15,Citation18–22 included only hemodialysis patients. Six studiesCitation15,Citation16,Citation19–22 made dietary recommendations or monitored the dietary phosphate intake for the participants. Most studies co-administered vitamin D compounds and some studies also co-administered erythropoietin and calcium supplementation.
Table 1. Characteristics of studies included in the meta-analysis.
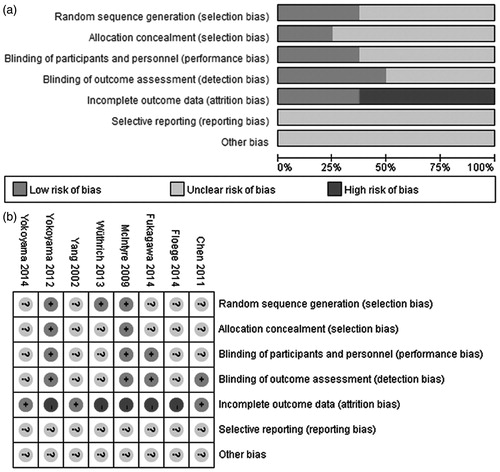
Risk of bias graph and risk of bias summary for included studies are shown in . Random sequence generations were adequate in three studies.Citation18,Citation19,Citation22 Allocation concealments were adequately described with low risk of bias in two studiesCitation18,Citation22 and unclear in other six studies. Participants and investigators were blinded in three studiesCitation17,Citation18,Citation22 and outcome assessors were blinded in four studies.Citation15,Citation17,Citation18,Citation22 The number of participants lost to follow-up ranged from 13.1 to 38.1% but did not differ between the treatment and control groups. Only three studiesCitation15,Citation18,Citation22 were analyzed on an intention-to-treat basis. Five studiesCitation17,Citation19–22 were supported by the production or sales manufacturer and declared conflicts of interest.
Iron-based phosphate binders versus placebo
Three studiesCitation17,Citation18,Citation22 compared iron-based phosphate binders with placebo. Of these studies, oneCitation18 compared fermagate with placebo, oneCitation22 made comparison between JTT-751 and placebo, and the other studyCitation17 compared SBR759 to placebo. Treatment duration ranged from 3 to 4 weeks and sample size varied from 63 to 192. The mean age of the participants ranged from 59.1 to 62.8 years.
Serum phosphorus
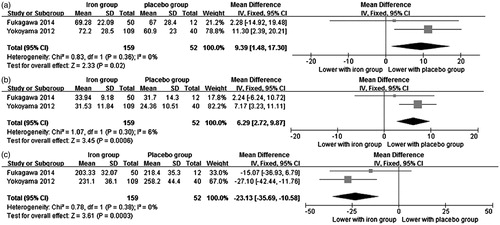
All studies reported the effect of iron-based phosphate binders on serum phosphorus (expressed as mean ± standard deviation) and were pooled in our analysis. Iron-based phosphate binders were associated with significant lower serum phosphorus levels in comparison to placebo (MD = −2.43 mg/dL, 95% CI −3.18 to −1.68, p < 0.00001) with significant heterogeneity among studies (Chi2 = 6.07, I2 = 67%) (). Exclusion of any single study did not change the result, with MD varied from −2.67 mg/dL (95% CI −3.80 to −1.55, p < 0.00001) to −2.10 mg/dL (95% CI −2.53 to −1.67, p < 0.00001). The three studies also reported the proportions of phosphate-controlled patients and the iron group was associated with higher proportion of phosphate-controlled patients compared with placebo group (OR = 14.25, 95% CI 2.72 to 74.72) with significant heterogeneity between studies (Chi2 = 5.48, I2 = 63%).
Serum calcium
In a study by McIntyre,Citation18 there was no change in mean serum calcium levels between the fermagate and the placebo treatment group. In another study by Yokoyama,Citation22 serum calcium increase significantly only in the JTT-751 6 g/day group (p < 0.001), but the value of serum calcium remained within the normal range. As the two studies did not report serum calcium as mean ± standard deviation and were not pooled in our meta-analysis.
Serum calcium × phosphorus product (Ca × P)
The effect of iron-based phosphate binders versus placebo in serum Ca × P product was reported only in one study.Citation17 The mean changes of serum Ca × P product levels from baseline were −26.29 mg2/dL2 in the SBR759 group compared with 2.02 mg2/dL2 in the placebo group.
Serum iPTH
In Fukagawa’s study,Citation17 the change of serum iPTH levels from baseline were −96.65 pg/mL for the SBR759 group compared with 37.63 pg/mL for the placebo group. In Yokoyama’s studyCitation22, the changes of serum iPTH levels from baseline between each of the JTT-751 groups and the placebo group showed a significant decrease in the 3 g/day and 6 g/day groups (p < 0.05 and p < 0.001). As the value of serum iPTH level was not reported as mean ± standard deviation and was not pooled in our meta-analysis.
Serum hemoglobin
Only one studyCitation22 reported the effects of iron-based phosphate binders versus placebo on serum hemoglobin. Iron-based phosphate binders were associated with significant higher serum hemoglobin levels in comparison to placebo (10.83 ± 1.07 g/dL vs. 10.27 ± 1.08 g/dL, p = 0.005).
Serum iron-related parameters
As shown in , iron-based phosphate binders were associated with significant higher serum iron (two studiesCitation17,Citation22, MD = 9.39 ng/mL, 95% CI 1.48 to 17.30, p = 0.02), higher serum TSAT (two studiesCitation17,Citation22, MD = 6.29%, 95% CI 2.72 to 9.87, p = 0.0006) and lower serum TIBC (two studiesCitation17,Citation22, MD = −23.13 µg/dL, 95% CI −35.69 to −10.58, p = 0.0003) in comparison to placebo. There was no significant difference between the two groups in serum ferritin (only one studiesCitation17, MD = 5.59 µg/dL, 95% CI −57.35 to 68.53, p = 0.86).
Adverse events
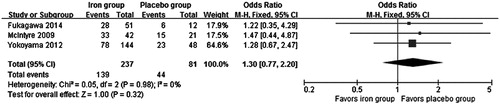
All the three studies reported adverse events of the two treatments in their study duration. No significant difference was found in all adverse events between the iron group and the placebo group (OR = 1.30, 95% CI 0.77 to 2.20, p = 0.32) and no significant heterogeneity was identified (Chi2 = 0.05, I2 = 0%) (). Exclusion of any single study did not change the result, with a range from 1.27 (95% CI 0.71 to 2.27, p = 0.42) to 1.34 (95% CI 0.56 to 3.20, p = 0.51). All of them reported the number of patients withdrawing from the trials due to adverse events and our analysis found no significant difference between the two treatment groups (OR = 1.58, 95% CI 0.59 to 4.22, p = 0.36). The incidence of adverse drug reaction was generally similar between the two treatment groups (two studiesCitation17,Citation18, OR = 1.55, 95% CI 0.45 to 5.31, p = 0.48). The most common adverse events of iron-based phosphate binders are gastrointestinal reactions, such as discolored feces, diarrhea, constipation, and abdominal distension. Other side-effects included nasopharyngitis, myalgia, and procedural hypotension. Most adverse events were mild and only two serious adverse events (chest pain and arteriovenous fistula occlusion) in one studyCitation18 were considered possibly related to the fermagate 2 g/day treatment.
All-cause mortality
Only one study reported 1 death in 63 participants, the death caused by acute myocardial infarction was in the placebo group, and was considered unrelated to the study medication.
Iron-based phosphate binders versus calcium-based phosphate binders
One studyCitation20 with 54 participants compared ferric citrate with calcium carbonate in hemodialysis patients in a cross-over randomized controlled trial. At week 4, serum phosphorus fell by approximately 2 mg/dL in calcium carbonate group and by 1 mg/dL in ferric citrate group (p < 0.0001). Serum calcium increased and serum iPTH decreased only in calcium carbonate treatment group. Serum Ca × P product declined during both treatments without significant difference between the two treatments. More patients in ferric citrate treatment group experienced adverse events than in calcium carbonate treatment group (26/54 vs. 8/54).
Iron-based phosphate binders versus sevelamer
Four studiesCitation15,Citation16,Citation19,Citation21 compared iron-based phosphate binders with sevelamer, one studyCitation21 compared JTT-751 with sevelamer, two studiesCitation16,Citation19 made comparison between PA21 and sevelamer, and the other studyCitation15 compared SBR759 to sevelamer. Treatment duration ranged from 6 to 24 weeks and sample size varied from 154 to 1059. The mean age of the participants ranged from 56 to 60.8 years.
Serum phosphorus
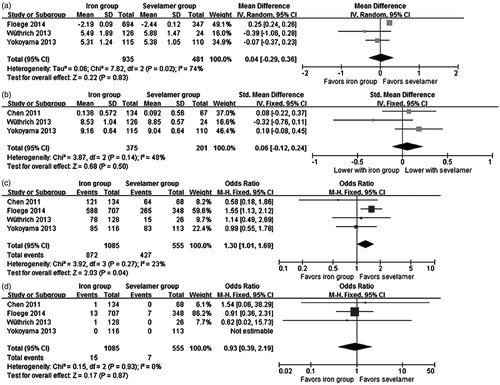
Three studiesCitation16,Citation19,Citation21 reported the effect of iron-based phosphate binders on serum phosphorus (expressed as mean ± standard deviation) and were pooled in our meta-analysis. There was no significant difference in serum phosphorus between iron-based phosphate binders and sevelamer (MD = 0.04 mg/dL, 95% CI −0.29 to 0.36, p = 0.83), with significant heterogeneity among studies (Chi2 = 7.82, I2 = 74%) (). Exclusion of any single study did not change the result, with a range from −0.12 (95% CI −0.40 to 0.15, p = 0.38) to 0.13 (95% CI −0.18 to 0.43, p = 0.42). Three studiesCitation15,Citation19,Citation21 reported the proportions of phosphate-controlled patients in each group, and no significant difference was found between the two treatments (OR = 1.18, 95% CI 0.85 to 1.64, p = 0.31) with significant heterogeneity among studies (Chi2 = 7.18, I2 = 72%).
Serum calcium
The effect of iron-based phosphate binders on serum calcium (expressed as mean ± standard deviation) was reported in three studies.Citation15,Citation19,Citation21 There was no significant difference between iron-based phosphate binders and sevelamer (SMD = 0.06 mg/dL, 95% CI −0.12 to 0.24, p = 0.50), with non-significant heterogeneity between studies (Chi2 = 3.87, I2 = 48%) ().
Serum calcium × phosphorus product (Ca × P)
In a study by Wüthrich et al.,Citation19 the changes in serum Ca × P product from baseline during the treatment were −10.69 ± 18.03 mg2/dL2 in the PA21 group and −9.71 ± 12.91 mg2/dL2 in the sevelamer group (p = 0.75). However, in Chen et al.’s study,Citation15 more patients achieved a Ca × P product ≤ 4.4 mmol2/L2 in the SBR759 group than in the sevelamer group (84.0% vs. 67.0%, p = 0.0173). Mean reduction in serum Ca × P product was also significantly greater with SBR759 than with sevelamer (−1.98 mmol2/L2 vs. −1.41 mmol2/L2, p = 0.0031). As the data of serum Ca × P product was not reported as mean ± standard deviation and was not pooled in our analysis.
Serum iPTH
In Chen et al.’s work,Citation15 no clinically significant change was observed in serum iPTH concentrations after 12 weeks of iron-based phosphate binders or sevelamer treatment. In Yokoyama’s study,Citation21 serum iPTH level at the end of treatment was significantly reduced from baseline in both groups (p < 0.001). But there was no significant difference between the two treatment groups (p = 0.73). In Wüthrich’s study,Citation19 the greatest mean decrease in serum iPTH was seen in the PA21 12.5 g/d group. However, there was no observed dose-dependent relationship. In Floege’s study,Citation16 the median serum iPTH concentrations decreased significantly from baseline to week 24 in both treatment groups, the decrease was more pronounced in the PA21 group (p = 0.04 for the comparison of the changes between groups). As there were no detailed data (expressed as mean ± standard deviation) in these studies, we did not pool them.
Serum hemoglobin
In Yokoyama’s study, JTT-751 was associated with higher hemoglobin (from 10.9 ± 1.0 to 11.8 ± 1.5, p < 0.001) than sevelamer (from 10.8 ± 1.1 to 10.6 ± 1.0, p = 0.0344). But in Floege’s, there were no significant changes in hemoglobin parameters in both the two groups without detail data.
Serum iron-related parameters
In Wüthrich’s study, there were no significant changes from baseline and no trends detected across treatment groups for serum iron. Serum iron values also remained unchanged in Chen’s work. But in Yokoyama’s, JTT-751 was associated with higher serum iron than sevelamer (p < 0.001).
In Chen’s study, serum ferritin increased in the SBR759 arm at the end of the treatment (from 480 to 608). In Floege’s study, median serum ferritin concentrations increased in both treatment groups. In Yokoyama’s study, serum ferritin increased significantly in the JTT-751 group (from 48.2 ng/mL to 123 ng/mL, p < 0.001) and decreased in the sevelamer group (from 66.9 ng/mL to 54.7 ng/mL, p = 0.0913). In Wüthrich’s, there was no difference across treatment groups for serum ferritin.
In Wüthrich’s study, there were no significant changes from baseline and no significant difference found across treatment groups for serum TIBC. Serum TIBC also remained unchanged in Chen’s work. But in Yokoyama’s, serum TIBC decreased significantly in the JTT-751 group (from 249 to 226.5, p < 0.001) and increased in the sevelamer group (from 241 to 261, p < 0.001).
In Wüthrich’s study, there were no significant changes from baseline and no trends detected across treatment groups for serum TSAT. The TSAT remained unchanged in both treatment arms in Chen’s work. In Floege’s, increases in TSAT were only seen with PA21. In Yokoyama’s, serum TSAT increased significantly in the JTT-751 group (from 23 to 35.9%, p < 0.001) and decreased in the sevelamer group (from 24.1 to 23.5%, p = 0.0093).
The data of serum iron-related parameters was reported in different measurements, such as mean value or median value, thus could not be pooled in our meta-analysis.
Adverse events
All the four studies reported adverse events in their treatment duration. Significant difference was found in all adverse events between the iron group and the sevelamer group (OR = 1.30, 95% CI 1.01 to 1.69, p = 0.04) and no significant heterogeneity was identified (Chi2 = 3.92, I2 = 23%) (). Exclusion of Floege’s work changed the result, with an OR of 0.94 (95% CI 0.61 to 1.47, p = 0.80), so the result should be interpreted with caution. All of them reported the number of patients withdrawing from the trials due to adverse events and our analysis found no significant difference between the two groups (OR = 1.04, 95% CI 0.35 to 3.09, p = 0.94) with significant heterogeneity (Chi2 = 15.70, I2 = 81%). Our analysis found that the incidence of adverse drug reaction was similar between the two groups (three studies,Citation15,Citation16,Citation21 OR = 1.21, 95% CI 0.48 to 3.04, p = 0.69) with significant heterogeneity (Chi2 = 21.87, I2 = 91%). The most common adverse events of iron-based phosphate binders are gastrointestinal reactions, such as discolored feces and diarrhea. Most adverse events were mild and no serious adverse event was considered related to the treatment drug.
All-cause mortality
Four studies with 22 deaths in 1640 participants (15 in the iron group and 7 in the sevelamer group) were pooled in our meta-analysis. One patient in the PA21 group died due to gastrointestinal hemorrhage and cardiac arrest, one death in the SBR759 group caused by myocardial infarction, and the deaths recorded in Floege’s study were mostly related to cardiac disorders. No significant difference was found in all-cause mortality (OR = 0.93, 95% CI 0.39 to 2.19, p = 0.87) between iron-based phosphate binders and sevelamer, with non-significant heterogeneity among the studies (Chi2 = 0.15, I2 = 0%) (). Further exclusion of any single study did not change the result, with a range from 0.89 (95% CI 0.36 to 2.17, p = 0.80) to 1.03 (95% CI 0.11 to 10.03, p = 0.98).
Discussion
To the best of our knowledge, this is the first study to evaluate the effects and safety of iron-based phosphate binders for hyperphosphatemia in dialysis patients. Eight studies with 2018 participants were involved in our review. The present review showed that iron-based phosphate binders were superior to placebo in reducing serum phosphorus in dialysis patients. Iron-based phosphate binders were as efficient as sevelamer for hyperphosphatemia with similar serum calcium level in dialysis patients. Iron-based phosphate binders were associated with higher serum iron, TSAT, and lower TIBC compared with placebo. In Yokoyama’s study, the median weekly erythropoiesis stimulating agents dose tended to decrease in the JTT-751 treatment group (recombinant erythropoietin product: p = 0.09, darbepoetin alpha: p < 0.001) compared with the sevelamer group. If such laboratory changes are confirmed over longer time periods and observational data regarding intravenous iron and erythropoiesis stimulating agent dose reduction hold true, iron-based phosphate binders could have a substantial clinical and economic benefit for renal anemia. Iron-based phosphate binders are very effective in small dosages, whereby patients experience a comparatively low tablet burden, which is conductive for therapy adherence. For other outcomes, data was not reported as mean ± standard deviation in these studies, and thus could not be pooled.
The incidence of all adverse events was similar between iron-based phosphate binders and placebo. The most common adverse events of iron-based phosphate binders are gastrointestinal reactions, such as discolored feces and diarrhea. Iron-based phosphate binders did not significantly reduce all-cause mortality compared with sevelamer in dialysis patients. Our conclusions are limited by the small number and short study duration of included studies. Indeed, we observed a trend towards a decrease of all-cause mortality in favor of iron-based phosphate binders, but the effects did not reach a statistical significance. It highlights the need for further larger-scale and in-depth randomized controlled trials to demonstrate.
In 2012, a meta-analysisCitation23 (an abstract) was published to assess the effects of ferric citrate on serum ferritin and TSAT in hemodialysis patients. This meta-analysis included only phase II randomized clinical studies and compared the effect of different doses of ferric citrate in serum iron-related parameters in hemodialysis patients. This meta-analysis showed that ferric citrate was associated with moderate dose-related increases in serum ferritin and TSAT with varying dosing schemes at day 28. The results were not completely consistent with ours and data on serum phosphorus, serum calcium, serum Ca × P product, serum iPTH, serum hemoglobin, adverse events, and all-cause mortality were not reported.
Our systematic review has several strengths. In an attempt to produce robust results, we formulated rigorous inclusion criteria in advance and included only randomized controlled trials that clearly stated the enrollment and allocation of patients receiving dialysis. Our study was based upon a broad systematic search of medical databases, data extraction, analysis, and study quality assessment by two independent review authors with supervision by a third experienced investigator. Further, while a number of studies did have a high loss to follow-up, the dropout between treatment groups was not different. Some limitations of this meta-analysis should be taken into account. First, the strength of conclusions drawn from this review is limited by the lack of long-term, large sample size, high quality studies. Three of eight studies enrolled fewer than 100 participants, all studies had a follow-up duration of less than 6 months and most of the included studies had a high or unclear risk of bias. Second, significant heterogeneity existed in several outcomes due to various kinds of iron-based phosphate binders, different doses of treatment medication, and different dialysis status. Third, two studies did not make dietary recommendations or monitored the dietary phosphate intake for the participants, which may potentially affect the effect of phosphate lowering between the two treatment groups.
For control of hyperphosphatemia, the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) recommends both calcium-based and calcium- and aluminum-free phosphate binders in patients receiving dialysis.Citation6 Therefore, the choice of phosphate binders should be individualized, considering the clinical context, the costs, the individual tolerability, and the concomitant effects on other parameters of mineral metabolism, such as serum calcium and iPTH, besides those on serum phosphorus.
Clearly, further trials are needed to be carried out in future to provide more reliable evidence. These trials should focus on the following points. First, large sample with long follow-up duration and high-quality randomized controlled trials are needed. Investigators should do their best to avoid all kinds of bias as much as possible in the design of randomized controlled trials. Second, more trials should be conducted in some other areas besides Asia. Although further development of SBR759 was recently terminated in Western patients due to less favorable efficacy and tolerability observed in a phase II study,Citation24 potent phosphate lowering capacity and good safety were observed with SBR759 in the study included in our review. Third, further studies should pay more attention to some meaningful outcomes, such as all-cause mortality, cardiovascular events, hospitalization, fibroblast growth factor-23, vascular calcification, and bone outcomes. Finally, more studies should focus on some other comparison, such as comparison between iron-based phosphate binders and calcium-based phosphate binders or between iron-based phosphate binders and lanthanum carbonate.
In conclusion, iron-based phosphate binders are as effective as sevelamer and well tolerated for hyperphosphatemia in dialysis patients. Iron-based phosphate binders appear to be a beneficial effect on renal anemia in patients receiving dialysis. Therefore, iron-based phosphate binders may represent a new treatment option for dialysis patients.
Supplementary material available online.
Supplemental Data
Download PDF (25.5 KB)Acknowledgments
The authors would like to acknowledge Shandong Provincial Hospital Library for contributions in search strategies and full-text retrieval.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165–180
- United States Renal Data System. 2013 annual data report: Atlas of end-stage renal disease. Costs of end-stage renal disease. Available at: http://www.usrds.org/2013/view/v2_11.aspx. Accessed February 12, 2014
- Bellasi A, Mandreoli M, Baldrati L, et al. Chronic kidney disease progression and outcome according to serum phosphorus in mild-to-moderate kidney dysfunction. Clin J Am Soc Nephrol. 2011;6(4):883–891
- Eddington H, Hoefield R, Sinha S, et al. Serum phosphate and mortality in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(12):2251–2257
- Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70(4):771–780
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl 3):S1–S201
- Yamada K, Fujimoto S, Nishiura R, et al. Risk factors of the progression of abdominal aortic calcification in patients on chronic hemodialysis. Nephrol Dial Transplant. 2007;22(7):2032–2037
- Kidney Disease: Improving Global Outcomes, C.K.D.M.B.D. Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;(113):S1–S130
- Oka Y, Miyazaki M, Takatsu S, et al. Sevelamer hydrochloride exacerbates metabolic acidosis in hemodialysis patients, depending on the dosage. Ther Apher Dial. 2007;11(2):107–113
- Dwyer JP, Sika M, Schulman G, et al. Dose-response and efficacy of ferric citrate to treat hyperphosphatemia in hemodialysis patients: A short-term randomized trial. Am J Kidney Dis. 2013;61(5):759–766
- Sinsakul M, Sika M, Koury M, et al. The safety and tolerability of ferric citrate as a phosphate binder in dialysis patients. Nephron Clin Pract. 2012;121(1–2):c25–c29
- Iida A, Kemmochi Y, Kakimoto K, et al. Ferric citrate hydrate, a new phosphate binder, prevents the complications of secondary hyperparathyroidism and vascular calcification. Am J Nephrol. 2013;37(4):346–358
- Block GA, Brillhart SL, Persky MS, Amer A, Slade AJ. Efficacy and safety of SBR759, a new iron-based phosphate binder. Kidney Int. 2010;77(10):897–903
- Geisser P, Philipp E. PA21: A novel phosphate binder for the treatment of hyperphosphatemia in chronic kidney disease. Clin Nephrol. 2010;74(1):4–11
- Chen JB, Chiang SS, Chen HC, et al. Efficacy and safety of SBR759, a novel calcium-free, iron(III)-based phosphate binder, in Asian patients undergoing hemodialysis: A 12-week, randomized, open-label, dose-titration study versus sevelamer hydrochloride. Nephrology (Carlton). 2011;16(8):743–750
- Floege J, Covic AC, Ketteler M, et al. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int. 2014;86(3):638--647
- Fukagawa M, Kasuga H, Joseph D, et al. Efficacy and safety of SBR759, a novel calcium-free, iron (III)-based phosphate binder, versus placebo in chronic kidney disease stage V Japanese patients on maintenance renal replacement therapy. Clin Exp Nephrol. 2014;18(1):135–143
- McIntyre CW, Pai P, Warwick G, Wilkie M, Toft AJ, Hutchison AJ. Iron-magnesium hydroxycarbonate (fermagate): A novel non-calcium-containing phosphate binder for the treatment of hyperphosphatemia in chronic hemodialysis patients. Clin J Am Soc Nephrol. 2009;4(2):401–409
- Wüthrich RP, Chonchol M, Covic A, Gaillard S, Chong E, Tumlin JA. Randomized clinical trial of the iron-based phosphate binder PA21 in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8(2):280–289
- Yang WC, Yang CS, Hou CC, Wu TH, Young EW, Hsu CH. An open-label, crossover study of a new phosphate-binding agent in hemodialysis patients: Ferric citrate. Nephrol Dial Transplant. 2002;17(2):265–270
- Yokoyama K, Akiba T, Fukagawa M, et al. A randomized trial of JTT-751 versus sevelamer hydrochloride in patients on hemodialysis. Nephrol Dial Transplant. 2014;29(5):1053--1060
- Yokoyama K, Hirakata H, Akiba T, Sawada K, Kumagai Y. Effect of oral JTT-751 (ferric citrate) on hyperphosphatemia in hemodialysis patients: Results of a randomized, double-blind, placebo-controlled trial. Am J Nephrol. 2012;36(5):478–487
- Bond C, Jensen D, Wang S, et al. A meta-analysis of ferric citrate for hyperphosphatemia: The effects of an oral iron-containing phosphate binder on serum ferritin and saturated transferrin in hemodialysis patients. Am J Nephrol. 2012;27:ii490
- Novartis Clinical Trial Results Database. A 12-week, open label, multicentre, titration study, with a 9-month maintenance treatment extension, to demonstrate efficacy of SBR759 compared to sevelamer-HCl in lowering serum phosphate levels in Chronic Kidney Disease patients on hemodialysis. Available at: http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do?trialResult=4664. Accessed May 25, 2014