Abstract
Aim: Neutrophil–lymphocyte ratio (NLR) is an easily calculated, sensitive, and accurate marker for prognosis and diagnosing sepsis, cardiovascular disease and cancer. As sepsis and septic shock are main causes of acute kidney injury (AKI) intensive care unit (ICU), we investigated whether NLR is an early predictor of AKI in patients with severe sepsis. We compared NLR's predictive power with that of other inflammation-related variables. Methods: Between December 2011 and November 2013, we enrolled 118 consecutive cases with severe sepsis admitted to ICU in this retrospective study. Levels of C-reactive protein (CRP), NLR, and white blood cell count (WBC) were recorded on admission and patients' renal function was monitored for seven consecutive days. Results: The rate of AKI occurrence 7 days after enrollment was 57.6%. NLR levels were higher in the AKI group (Group 1) than in the non-AKI group (Group 2) on the day of ICU admission (p < 0.001). AKI development was independently associated with NLR, Acute Physiology and Chronic Health Evaluation II (APACHE II) and duration of invasive mechanical ventilation (MV) in multivariate logistic regression analysis. The area under the receiver-operating characteristic (ROC) curve of NLR for predicting AKI was 0.986, which was superior to WBC and CRP (p < 0.05). The cut-off value of 10.15 for NLR had the highest validity for predicting AKI in patients with severe sepsis. The sensitivity, specificity, negative-predictive value (NPV), and positive-predictive value (PPV), for this cut-off value was 90.2%, 92.9%, 90.4%, and 92.7%, respectively. Conclusion: NLR is superior to CRP, and WBC for predicting the development of AKI in patients with severe sepsis.
Introduction
Acute kidney injury (AKI) is a common sequel of sepsis and is associated with unacceptably high morbidity and mortality, prolonged hospital stay, and increased costs of care among critically ill patients.Citation1,Citation2 AKI rates in patients with septic shock have been reported to be between 51% and as high as 65% in intensive care units (ICUs).Citation3,Citation4 Despite AKI have multiple components of: ischemia-reperfusion injury, direct inflammatory injury, coagulation and endothelial cell dysfunction, and apoptosis; the exact pathophysiology of sepsis-induced AKI remains unknown.Citation5–7
The predictors of AKI have not been well characterized in critically ill patients with sepsis. Creatinine are usually used as traditional blood marker of kidney injury, are insensitive for the early diagnosis of AKI.Citation8–10 Several urinary biomarkers aid diagnosis of AKI earlier than a rise in serum creatinine.Citation8,Citation9 But they are too expensive, their measurements are not possible routinely nowadays and combining multiple biomarkers is more sensitive for early detection of AKI than single biomarker.Citation8–10
There is now increasing experimental and clinical studies for an undeniable role of inflammation in the pathophysiology of AKI.Citation5–7 Development of inflammation could be caused by local inflammation of kidney tissues.Citation11,Citation12 There is typically a systemic inflammation during AKI.Citation5,Citation7,Citation13–17
Neutrophil–lymphocyte ratio (NLR) is an indicator of systemic inflammation, calculated easily from complete blood count.Citation18 Some studies showed that NLR is promising marker in predicting bacteremia in patients with emergency medical admissions.Citation19–21 NLR was used as prognostic marker in patient with bacteremia.Citation20
We aimed to investigate whether NLR is a predictor of AKI in patients with septic shock.
Materials and methods
We performed a retrospective cohort analysis of consecutive adult (>18 years of age) patients with a first episode of severe sepsis admitted to a medical intensive care unit (ICU) in a tertiary care hospital from December 2011 until November 2013. This study was approved by Local ethics committee (IRB number: 99950669/168). No additional informed consent was required.
The severity of illness was evaluated according to the Sequential Organ Failure Assessment (SOFA) score.Citation22 The SOFA score included six systems which were respiratory, cardiovascular, neurologic, renal, hematologic, and hepatic system. SOFA scores were calculated from the data available at the onset of septic shock. Acute Physiology and Chronic Health Evaluation II (APACHE II) scores were determined in all patients after the first 24 h of admission.Citation23 Each case of severe sepsis was defined according to International Sepsis Definitions Conference criteria.Citation24 Patients with severe sepsis had to meet at least one of the following nine criteria in addition to sepsis:Citation24
Sepsis-induced hypotension.
Lactate above upper limits laboratory normal.
Urine output <0.5 mL/kg/h for more than 2 h despite adequate fluid resuscitation.
Acute lung injury with Pao2/Fio2 < 250 in the absence of pneumonia as infection source.
Acute lung injury with Pao2/Fio2 < 200 in the presence of pneumonia as infection source.
Creatinine >2.0 mg/dL (176.8 μmol/L).
Bilirubin >2 mg/dL (34.2 μmol/L).
Platelet count <100,000 μL.
Coagulopathy (international normalized ratio >1.5).
The study cohort consisted of all patients who had positive blood cultures obtained upon presentation at the ICU. For our study, exclusion criteria: (1) admission renal Sequential Organ Failure Assessment (SOFA) scoreCitation22 was 1 or more (i.e., baseline creatinine ≥1.2 mg/dL); (2) renal replacement therapy (RRT) was used at the start of the study; (3) there was absence of Scr measures during the first week after baseline; (4) patients with hematological disease, receiving chemotherapy or glucocorticoids; (5) patients in whom care was withdrawn within 6 h of onset of septic shock; (6) prerenal and postrenal causes of AKI; (7) being exposed to radiocontrast dye or nephrotoxic drugs (aminoglycoside, colistin, and amphotericin) within at least one week before the admission to ICU; (8) there was other causes of lymphocytopenia such as corticosteroid use, HIV infection, malnutrition, systemic lupus erythematosus, rheumatoid arthritis, sarcoidosis, cytotoxic agents, immunosuppressive drugs, some malignancies, and radiation.
AKI was defined as an increase in the serum creatinine concentration of ≥0.3 mg/dL from baseline; a percentage increase in the serum creatinine concentration of ≥50%; or oliguria of <0.5 mL/kg/h for more than six hours during the first week after baseline.Citation25 AKI should be both abrupt (within 1–7 days) and sustained (more than 24 h).
Upon admission into the ICU, the following items were recorded for each patient from database: age, gender, body mass index, smoking, APACHE II scores, SOFA scores, serum creatinine, urea, aspartate aminotransferase (AST), alanine aminotransferase (ALT), calcium, Na, K, albumin, uric acid, serum c-reactive protein (CRP), urine output, MV (its duration), etiological factors, and underlying diseases.
Within 24 h after ICU admission, complete blood count was used to calculate NLR (a simple ratio between the absolute neutrophil and the absolute lymphocyte counts) and also to determine white blood cell (WBC)–platelet counts output from a standard Coulter® counter (LH 780 Hematology Analyzer, Beckman Coulter, Inc., Brea, CA).
The Kolmogorov–Smirnov test was used to test for normal distribution. All continuous data are presented as means ± SD or medians (interquartile range, IQR), as appropriate for nonparametric or parametric data, respectively. Categorical data are summarized as counts with percentages. Categorical variables were compared using the chi-squared test and continuous variables were compared using the Mann–Whitney U-test or Student’s t-test. We performed stepwise multivariate logistic regression analyses to assess the association of clinical and laboratory markers with the binary presence of AKI (yes/no). Odds ratios (ORs) and 95% confidence intervals were calculated. To be entered into this model, a p < 0.05 from univariate analysis was required. Receiver operating characteristic (ROC) curves was constructed to evaluate the sensitivity and specificity of the WBC, NLR, and CRP levels in predicting AKI in severe sepsis. p < 0.05 was considered to represent a statistically significant difference. All analyses were performed using SPSS software (version 17.0; SPSS Inc., Chicago, IL).
Results
One hundred-eighteen patients, who had suitable criteria, were included to this study. All datas were recorded exactly from our database during seven days after inclusion. We classified into two groups. Group 1 (n = 68) was composed of severe sepsis patients with developing AKI and Group 2 (n = 50) was composed of severe sepsis patients without developing AKI.
Demographic features, clinical and laboratory data of the patients are presented in . Group 1 had more advanced age than Group 2 (63.7 ± 12.2 vs. 54 ± 9.8, p < 0.001). There was no difference in gender, and body-mass index, albumin, CRP, WBC between Group 1 and Group 2. Laboratory data showed that patients in Group 1 had higher levels of NLR compared to patients in Group 2 (13.56 ± 4.39 vs. 7.83 ± 2.45; p < 0.001).
Table 1. Patients’ characteristics and clinical outcome.
Multivariate logistic regression was used to assess possible risk factors for AKI occurrence. All variables were taken into consideration here. But, from univariate analyses, five variables were selected for multivariate regression: APACHE II, NLR, use of ACE or ARB, duration of invasive MV and global SOFA score. Age was added to multivariate logistic analysis. After adjustment for confounders, the development of AKI was independently associated with NLR (OR: 3.25, CI: 2.72–4.19, p < 0.001), APACHE II (OR: 1.72 CI: 1.61–1.92, p = 0.038) and duration of invasive MV (OR: 1.28, per one hour, CI: 1.07–1.41, p = 0.048) ().
Table 2. Risk factors for development of AKI in patients with severe sepsis: multiple logistic regression analysis.
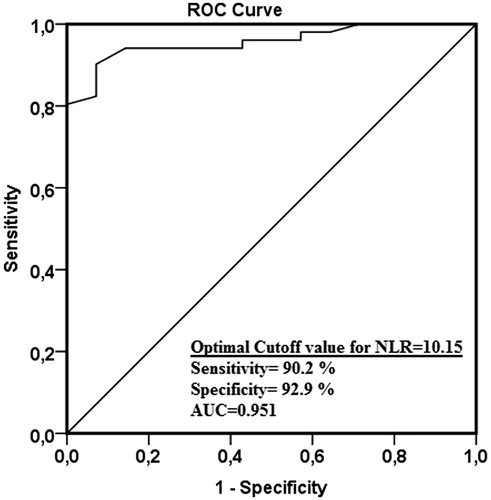
ROC curves are used for the prediction of AKI for WBC, NLR () and CRP levels. AUC for the prediction of AKI was 0.463 for WBC, 0.951 for NLR, and 0.531 for CRP (). Threshold level, sensitivity, and specificity of test could not be determined for WBC and CRP. Using a cut-off point of 10.15 for NLR, the sensitivity turns out 90.2% and the specificity 92.9%.
Table 3. Threshold levels, sensitivity and specificity of WBC, CRP and NLR levels for predicting AKI development.
Discussion
The study showed mainly that, within 24 h after ICU admission, calculated NLR is powerful and independent predictor of sepsis-related-AKI in severe sepsis. Patients with sepsis-related-AKI had higher NLR levels than patients without sepsis-related-AKI. If baseline NLR value was 10.15 or higher in severe sepsis, we would keep development of sepsis-related-AKI in mind. Secondly, duration of invasive MV, APACHE II score may be an independent predictor of sepsis-related-AKI.
The pathogenesis of AKI in patients who are critically ill with sepsis is scarce.Citation6 Throughout the past half century, septic AKI has traditionally been considered secondary to kidney ischemia as a consequence of decreased cardiac output and hypotension, which trigger sustained renal vasoconstriction and in turn exacerbate and sustain the ischemia.Citation6 Recent evidence suggests that sepsis-related-AKI is much more complex and may have a distinct pathophysiology from non-septic AKI.Citation6,Citation7 Ischemia-reperfusion injury, inflammation, microvascular alterations, and apoptosis are main mechanism for sepsis-related-AKI.Citation7 It is known that inflammation is a crucial role in the development of AKI;Citation6,Citation7,Citation26 systemic inflammation could be secondary to local kidney inflammationCitation11,Citation12 and systemic inflammation accompanied during AKI.Citation7,Citation13–17 Human studies showed that AKI is associated with organ failures and cytokine activation.Citation27 In addition, the relationship between inflammation and AKI severity is most powerful.Citation27 Sustained systemic inflammation appears to be related to development of AKI as well as other organ failures.Citation27 NLR could be used to evaluate the systemic inflammation.Citation18 It has been still unknown whether baseline NLR is a predictor of sepsis-related-AKI in patients with severe sepsis. Experimental studies showed that TNF-α plays a role in the early pathogenesis of sepsis-related-AKI.Citation28,Citation29 Clinical studies revealed that IL-6, procalcitonin (PCT) can be used as a predictive marker for sepsis-related-AKI in patients with sepsis.Citation30,Citation31 In accordance with these studies, we showed that baseline NLR is fruitful and strong predictor of sepsis-related-AKI. Urine NGAL, L-type fatty acid-binding protein (L-FABP) is new and useful markers for early detection of AKI in critically ill adults with an eGFR over 60 mL/min per 1.73 m2.Citation10 But these markers are very expensive to be used routinely, the use of multiple biomarkers are more reliable for early detection of AKI in patients with sepsis instead of single biomarker.Citation8–10 However, baseline NLR is cheap and has ability to predict the sepsis-related-AKI.
We found that CRP and WBC level on admission was not able to predict AKI in patients with severe sepsis, and ROC analysis revealed that the initial levels of these variables also performed poorly in predicting AKI compared to NLR levels. Some evidence suggested that high level of CRP was related to sepsis and mortality of critical illness.Citation32 However, one study demonstrated that CRP was not a predictor of AKI in patients with sepsis.Citation33 So to predict AKI in patients with severe sepsis, we recommended calculated NLR levels rather than simultaneously determining other inflammatory markers as well.
We found that duration of invasive MV is independent predictor of AKI in severe sepsis. Recently, one meta-analysis revealed that invasive mechanical ventilation is commonly accepted to be a risk factor for AKI in the critically ill and various methods of MV did not modify the risk.Citation34 Our results were consistent with this meta-analysis. We suggested that invasive MV contributed to the development of AKI in critically ill patients with severe sepsis via inflammation and its mediators.
APACHE II was used extensively to measure the severity of disease and predict mortality for adult patients admitted to intensive care units. Previously, two studies showed that APACHE II score aids to predict AKI in patients with sepsis.Citation30,Citation35 As expected, APACHE II was a predictor of sepsis-related-AKI in our study. Because APACHE II score contains 12 routine physiological components, no single parameter of APACHE II could predict sepsis-related-AKI according to our results. But global score of APACHE II had ability to predict sepsis-related-AKI. In this study, APACHE II was superior to SOFA for development of AKI in severe sepsis. The APACHE II scoring system uses the worst physiologic values measured within 24 h of admission to the ICU. SOFA uses data collected 24 h after admission and every 48 h thereafter. But both of the two scores were calculated within 24 h of admission to the ICU in this study. As it is known, initial SOFA score was inferior to maximum SOFA in the clinical course for prediction of mortality. We did not do serial assessment of SOFA score thus SOFA score cannot predict sepsis-related-AKI.
This study has some limitations. First, we are aware of the limitations of a retrospective study, which were difficult to control bias and confounders: no randomization, no blinding and we did not establish cause and effect. Second, the other limitation of the study was relatively small sample of our hospital. Third, initial SOFA score was recorded, although serial assessment of SOFA could be more appropriate. Nevertheless, our findings need to be validated by randomized prospective studies.
Conclusion
Baseline NLR would be a strong candidate for prediction of AKI in patients with severe sepsis. We suggested that cellular immunity and inflammation play a central role in prediction of AKI in severe sepsis. We considered that baseline NLR might be used to early diagnosis of AKI in severe sepsis if more prospective studies with larger sample sizes would be needed to confirm. We suggested that NLR, APACHE II and invasive mechanical ventilation appeared to be a risk factor for development of AKI in severe sepsis.
Declaration of interest
We have no conflict of interest and we have no funding, grant and financial support.
References
- Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370
- Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–973
- Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the Systemic Inflammatory Response Syndrome (SIRS): A prospective study. JAMA. 1995;273:117–123
- Bagshaw SM, Lapinsky S, Dial S, et al.; Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Acute kidney injury in septic shock: Clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 2009;35:871–881
- Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121(11):4210–4221
- Wan L, Bagshaw SM, Langenberg C, Saotome T, May C, Bellomo R. Pathophysiology of septic acute kidney injury: What do we really know? Crit Care Med. 2008;36(Suppl. 4):S198–S203
- Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7(4):189–200
- Slocum JL, Heung M, Pennathur S. Marking renal injury: Can we move beyond serum creatinine? Transl Res. 2012;159(4):277–289
- Siew ED, Ware LB, Ikizler TA. Biological markers of acute kidney injury. J Am Soc Nephrol. 2011;22(5):810–820
- Siew ED, Ware LB, Bian A, et al. Distinct injury markers for the early detection and prognosis of incident acute kidney injury in critically ill adults with preserved kidney function. Kidney Int. 2013;84(4):786–794
- Kramer AA, Postler G, Salhab KF, Mendez C, Carey LC, Rabb H. Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int. 1999;55:2362–2367
- Maitra R, Grigoryev DN, Bera TK, Pastan IH, Lee B. Cloning, molecular characterization, and expression analysis of Copine 8. Biochem Biophys Res Commun. 2003;303:842–847
- Lemay S, Rabb H, Postler G, Singh AK. Prominent and sustained up-regulation of gp130-signaling cytokines and the chemokine MIP-2 in murine renal ischemia-reperfusion injury. Transplantation. 2000;69:959–963
- Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238
- Rabb H, Daniels F, O’Donnell M, et al. Pathophysiological role of T lymphocytes in renal ischemiareperfusion injury in mice. Am J Physiol Renal Physiol. 2000;279:F525–F531
- Zhang Y, Woodward VK, Shelton JM, et al. Ischemia-reperfusion induces G-CSF gene expression by renal medullary thick ascending limb cells in vivo and in vitro. Am J Physiol Renal Physiol. 2004;286:F1193–F1201
- Kitada H, Sugitani A, Yamamoto H, et al. Attenuation of renal ischemiareperfusion injury by FR167653 in dogs. Surgery. 2002;131:654–662
- Bhat T, Teli S, Rijal J, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: A review. Expert Rev Cardiovasc Ther. 2013;11(1):55–59
- de Jager CP, van Wijk PT, Mathoera RB, de Jongh-Leuvenink J, van der Poll T, Wever PC. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care. 2010;14(5):R192
- Terradas R, Grau S, Blanch J, et al. Eosinophil count and neutrophil-lymphocyte count ratio as prognostic markers in patients with bacteremia: A retrospective cohort study. PLoS One. 2012;7(8):e42860
- Loonen AJ, de Jager CP, Tosserams J, et al. Biomarkers and molecular analysis to improve bloodstream infection diagnostics in an emergency care unit. PLoS One. 2014;9(1):e87315
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–829
- Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31
- Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. doi:10.1155/2009/137072
- Murugan R, Karajala-Subramanyam V, Lee M, et al. Genetic and Inflammatory Markers of Sepsis (GenIMS) Investigators. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77(6):527–535
- Knotek M, Rogachev B, Wang W, et al. Endotoxemic renal failure in mice: Role of tumor necrosis factor independent of inducible nitric oxide synthase. Kidney Int. 2001;59(6):2243–2249
- Bhargava R, Altmann CJ, Andres-Hernando A, et al. Acute lung injury and acute kidney injury are established by four hours in experimental sepsis and are improved with pre, but not post, sepsis administration of TNF-α antibodies. PLoS One. 2013;8(11):e79037. doi:10.1371/journal.pone.0079037
- Chawla LS, Seneff MG, Nelson DR, et al. Elevated plasma concentrations of IL-6 and elevated APACHE II score predict acute kidney injury in patients with severe sepsis. Clin J Am Soc Nephrol. 2007;2(1):22–30
- Nie X, Wu B, He Y, et al. Serum procalcitonin predicts development of acute kidney injury in patients with suspected infection. Clin Chem Lab Med. 2013;51(8):1655–1661
- Gaini S, Koldkjaer OG, Pedersen C, Pedersen SS. Procalcitonin, lipopolysaccharide-binding protein, interleukin-6 and C-reactive protein in community-acquired infections and sepsis: A prospective study. Crit Care. 2006;10(2):R53
- Mårtensson J, Bell M, Oldner A, Xu S, Venge P, Martling CR. Neutrophil gelatinase-associated lipocalin in adult septic patients with and without acute kidney injury. Intensive Care Med. 2010;36(8):1333–1340
- van den Akker JP, Egal M, Groeneveld JA. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: A systematic review and meta-analysis. Crit Care. 2013;17(3):R98
- Iglesias J, Marik PE, Levine JS. Elevated serum levels of the type I and type II receptors for tumor necrosis factor-alpha as predictive factors for ARF in patients with septic shock. Am J Kidney Dis. 2003;41:62–75