Abstract
Background: Patients with chronic kidney disease (CKD) present a markedly increased cardiovascular (CV) morbidity and mortality since the early stages and have a high prevalence of accelerated atherosclerosis, inflammation and endothelial dysfunction. Nontraditional cardiovascular risk factors and serum cardiac biomarkers would contribute to explain this increased morbidity. Aim: The aim is to investigate the relation among serum cardiac biomarkers (N-terminal pro-brain natriuretic peptide (NT-proBNP), cardiac troponin T (cTnT), nontraditional cardiovascular risk factors (serum uric acid, homocysteine), inflammatory indexes (C-reactive protein (CRP) serum ferritin, fibrinogen) and noninvasive predictors of atherosclerosis (carotid intima-media thickness (cIMT), brachial artery flow mediated dilation (baFMD), and left ventricular mass index (LVMI)) in CKD patients. Materials and methods: In 50 patients with CKD in stage 2/3 kidney disease outcomes quality initiative (KDOQI) and 18 age- and sex-matched healthy controls, the following parameters were measured: cardiac markers (cTnT and NT-proBNP), renal function, inflammatory markers (CRP, serum ferritin and fibrinogen), serum uric acid and homocysteine. We have also evaluated LVMIs, cIMT and baFMD. Results: In our study, we showed an increase of NT-proBNP and the serum cTnT, of serum uric acid and homocysteine with a positive correlation with the increase of cIMT and LVMI and reduced baFMD compared with the controls. Conclusions: Serum cardiac biomarkers and nontraditional cardiovascular risk factors increase already in the stage 2/3 KDOQI contributing to explain the high cardiovascular morbidity and mortality of these patients. The NT-proBNP seems to have a rise earlier compared with serum cTnT; however, both seemed to be a useful clinical biomarker for evaluating noninvasive predictors of atherosclerosis in CKD patients.
Introduction
Cardiovascular diseases (CVDs) are the leading cause of morbidity and mortality in patients with chronic kidney disease (CKD). Although traditional risk factors are common among these patients, they can only in part explain the increased susceptibility to CVDs.Citation1,Citation2 Cardiovascular risk factors promote the development of endothelial dysfunction that has a central role in the pathogenesis of CVDs together with inflammation, atherosclerosis, and mineral bone disease. Indeed vascular calcification and left ventricular hypertrophy (LVH) are the most commonly encountered clinical challenges and the most prevalent reasons for morbidity and mortality.Citation3–5 Nontraditional risk factors, such as hyperhomocysteinemia and hyperuricemia, have a high prevalence in renal disease and are associated with endothelial dysfunction and vascular damage.Citation6–11 Therefore, the identification of early markers of CVDs in this population is an important task for nephrologists. In fact, the study of Go et al.Citation12 shows an increased risk of death, cardiovascular events, and hospitalization in a large, community-based population already in the early stages of CKD. These findings highlight the clinical and public health importance of CKD. Cardiac troponin T (cTnT) is a sensitive and specific marker of ischemic myocardial damage, renally cleared, and widely used as a predictor of cardiovascular events.Citation13,Citation14 Natriuretic peptides, including brain natriuretic peptide (BNP) and its equimolar secreted N-terminal fragment prohormone brain natriuretic peptide (NT-proBNP), are increased in relation to cardiac stretch and LVH and established biomarkers for the diagnosis and risk stratification of patients with CVDs and heart failure. In the past, it has been suggested that in subjects with CKD, NT-proBNP and serum cTnT levels may be falsely elevated because of decreased renal clearance, even when there are no overt signs of volume overload, LVH, or coronary artery stenosis.Citation15 Instead, the predictive value of cTnT and NT-proBNP for cardiovascular events has been shown to be maintained in such subjects and reflect the subclinical cardiac damage. BNP was originally isolated from porcine brain but, in humans, is exclusively synthesized in the heart, and its synthesis and release are controlled at the level of gene expression, which is predominantly determined by ventricular hypertrophy and inflammation or in response to ventricular wall stress and filling pressure. The source of BNP and NT-proBNP is mainly the left ventricular and atrial myocytes.Citation15,Citation16 Distention of cardiac ventricle is considered the main stimulus for the release of proBNP1-108. This prohormone is released into the circulation and is proteolytically cleaved into the biologically active BNP1-32 and the inactive NT-proBNP1-76. The understanding of the cleavage of proBNP in circulation is most likely by the pro-protein convertases corrin and furin. BNP exerts its biological actions through its primary signaling receptor, guanylyl cyclase A. Several studies have demonstrated that there are only small quantities of intact BNP in the plasma, and the major circulating forms of BNP are degradation products.Citation13,Citation15 The clearance mechanism of BNP is through the endocytosis followed by lysosomal degradation and is degraded by a neutral endopeptidase that is abundantly expressed on cell surfaces. NT-proBNP is a biologically inert peptide that is cleared passively by organs with high rate of blood flow (muscle, liver, and kidney) and not by receptor-mediated mechanisms. Both are released in a 1:1 ratio, but NT-proBNP has a longer half-life (1–2 h) than BNP (15–20 min) and higher plasma concentration, also because of the greater stability. BNP and NT-proBNP tend to be higher in women and lower in obese individuals.Citation15,Citation17,Citation18 They are also higher in the elderly and in ventricular tachycardia, right ventricular overload, renal dysfunction, hypoxemia, myocardial ischemia, liver cirrhosis, infection, and sepsis. Despite the notable differences between the two peptides, both have been proven to be useful biomarkers for the diagnosis and risk stratification of heart failure, even if the ideal natriuretic peptide to diagnose heart failure in CKD remains undecided.Citation19,Citation20
Aim
This paper aims to study the relation among renal function, serum cardiac biomarkers (including NT-proBNP and cTnT), nontraditional risk factors (serum uric acid, homocysteine and C-reactive protein (CRP), serum ferritin, and fibrinogen), and noninvasive predictors of atherosclerosis (including carotid intima-media thickness (cIMT), brachial artery flow mediated dilation (baFMD), and echocardiography) in CKD patients.
Materials and methods
Study design and subjects
The study protocol was approved by the clinical research ethics committee of Sapienza University of Rome.
The study comprised 50 patients with CKD in stage 2/3 kidney disease outcomes quality initiative (KDOQI), 27 female and 23 male subjects with a median age of 52.6 years and 18 age- and sex-matched healthy controls. shows the patient characteristics. The estimated glomerular filtration rate (eGFR) was calculated with the abbreviated modification of diet in renal disease formula, as defined by Levey et al. The etiology of CKD in these patients was probable nephrosclerosis in 17cases, chronic glomerulonephritis in 8 cases, reflux nephropathy in 7 cases, chronic pyelonephritis in 3 cases, autosomal polycystic kidney disease in 4 cases, and unknown in 11 cases. Antihypertensive, antiplatelet, and statin therapies were continued in the patients included in the study. We recorded the cardiovascular history and excluded patients with heart failure, underlying malignancy, chronic liver disease, chronic obstructive airway disease, congenital heart disease, atrial fibrillation, cerebrovascular disease, common carotid artery stenosis, hypertension with poor blood pressure control, acute myocardial infarction, valvular heart disease, and acute coronary syndrome within 3 months before the study; patients who refused to give consent; and patients with missing data.
Table 1. Data are show as mean ± standard deviation, median (min–max) or number (%).
Laboratory measurements
Blood was drawn in the morning after an overnight fast of at least 12 h. In all the patients, the levels of fasting plasma glucose (mg/dL), hemoglobin (g/dL), serum total cholesterol (mg/dL), triglycerides (mg/dL), high-density lipoprotein (HDL) (mg/dL), low-density lipoprotein (LDL) (mg/dL), creatinine (mg/dL), azotemia (mg/dL), calcium (mg/dL), phosphorus (mg/dL), Ca*P (mg/dL), natrium (mEq/L), potassium (mEq/L), CRP (mg/dL), homocysteinemia (μmol/L), serum iron (µg/dL), serum ferritin (ng/mL), serum transferrin (mg/dL), and fibrinogen (mg/dL) were measured using standard automated techniques. NT-proBNP (pg/dL) and cTnT (ng/dL) were measured using automated analyzer Elecsys® 2010 (Roche Elecsys 2010 chemistry analyzer, Cobas Integra 400 Plus Analyzer, Geislingen, Germany). Renal function was estimated using the MDRD formula.
Anthropometric assessments
Body weight was determined to the nearest 0.1 kg using a calibrated digital scale. Body mass index was calculated from a person's weight and height (weight (kg)/[height (m)]2).
Blood pressure measurements
Clinic BP measurements were made three times after 10 min of rest in a seated position using a standard sphygmomanometer and cuffs adapted to the arm circumference according to the British Hypertension Society guidelines,Citation21 and the mean values for systolic BP (SBP) and diastolic BP (DBP) were calculated for all the participants. The systolic and diastolic BP levels were taken as the points of appearance and disappearance of Korotkoff sounds, respectively. Hypertension was defined as SBP >140 mmHg or DBP >90 mmHg on repeated measurements.
Common carotid intima-media thickness assessment
Common carotid artery imaging was performed by a single investigator in the vascular laboratory suite under standardized conditions with the high-resolution B-mode ultrasound machine Toshiba AplioxV (Toshiba AplioxV; Toshiba American Medical Systems, Inc., Tustin, CA) equipped with a 5- to 12-MHz linear transducer with a 0.01-mm resolution, following a standardized vascular protocol. Three different longitudinal views (anterior oblique, lateral, and posterior oblique) and a transverse view were obtained. Intima media thickness (IMT) was measured at three points on the far walls of both left and right distal common carotid arteries, carotid bulb, and proximal portion of the internal carotid arteries. Images were captured in end diastole triggered by electrocardiographic recording. The mean IMT was computed as the average IMT on both sides. The value of IMT was considered normal when between 0.55 and 1 mm.Citation22 The images were analyzed by a technician blinded to patient identity and study group.
Flow-mediated dilation brachial artery (baFMD)
According to the method described by Celermajer and others,Citation23 the endothelium-dependent vasodilation (FMD) of the brachial artery was assessed using high-resolution ultrasound.Citation24 The subjects were observed by a single investigator, and Toshiba AplioxV equipped with a 5- to 12-MHz linear transducer with a 0.01-mm resolution was used for the ultrasonographic study of FMD, following a standardized vascular protocol. The brachial artery was imaged above the antecubital fossa in the longitudinal plane. A segment with clear anterior and posterior intimal interfaces between the lumen and vessel wall was selected for continuous 2D gray-scale imaging. To create a flow stimulus in the brachial artery, a sphygmomanometric cuff was initially placed on the forearm. Typically, the cuff was inflated to at least 50 mmHg above the SBP to occlude the arterial inflow for a standardized length of time. The release of the occluding cuff resulted in reactive hand hyperemia and an associated increase in the blood flow through the brachial artery, which induced shear stress on the arterial wall and provided a stimulus for endothelium-dependent dilatation. Brachial artery diameter following reactive hyperemia was recorded for 5 min after tourniquet release. Flow-mediated vasodilatation was typically expressed as the change in poststimulus diameter as a percentage of the baseline diameter.Citation24
The values of FMD were considered normal if they were greater than 10%.
Echocardiography
M-mode 2D echocardiographic examinations by a single experienced sonographer in the echocardiography laboratory and using a standard institutional protocol were completed. Commercially available instruments Toshiba AplioxV equipped with 2.25- to 7.5-MHz imaging transducers were used; the subjects were in the left decubitus position, and the sonographer was blinded to all the clinical details of the patients. All echocardiographic data according to the guidelines of the American Society of Echocardiography (ASE) were recorded.Citation25 The end-diastolic and end-systolic left ventricular internal diameter (LVED and LVES, respectively), interventricular septum thickness (IVST), and posterior wall thickness (PWT) were measured.Citation26 The left ventricular mass (LVM) by Devereux's formulaCitation27 normalized by body surface area (BSA) and height was estimated.
Statistical analysis
Data management and analysis were performed using IBM® SPSS® Statistics 18 software for Windows® (IBM Corporation, Armonk, NY). The normality of the variables was tested using the Kolmogorov–Smirnov test for normal distributions. All continuous variables following a normal distribution were expressed as mean ± standard deviation. Categorical variables were expressed as number (percentage). Pearson's or Spearman's correlation was used to determine the relation and strength of the association between the variables. Univariate and multivariate linear regression was performed between FMD, IMT, or LVMI and parametric variables that reached statistical significance (p < 0.05) in the bivariate correlation. Multivariate linear regression analysis was performed to determine the independent correlations between FMD, IMT, or LMVI and other variables using a stepwise approach.
Results
presents the general characteristics of the study participants.
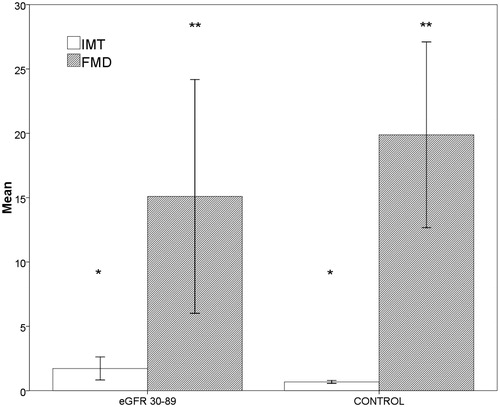
There were no statistically significant differences in age, sex, height (cm), weight, and BMI (kg/m2) between the groups. The linear regression multiple analysis showed an independent correlation between the decline in eGFR and increase in the values of NT-proBNP (β = −0.227, p = 0.012). The increase in the left ventricular mass correlated with the reduction of eGFR (r = −0.489, p < 0.001) () and with the increase of NT-proBNP (r = −0.491, p < 0.017) (). The median values of NT-proBNP were significantly different between groups (p < 0.001) (). The mean values of cIMT, homocysteine, uric acid, and Ca*P increased, whereas the mean baFMD values was reduced significantly in CKD versus controls (cIMT: p < 0.001; homocysteine: p < 0.001; serum uric acid: p < 0.022; Ca*P: p < 0.001; FMD: p < 0.048) (). There are statistically significant differences in the values of NT-proBNP in the early stages of CKD, although there are no statistically significant differences in the values of cTnT in the early stages (p = 0.414). The median values of cTnT were statistically significant when comparing patients with GFR >60 mL/min versus GFR <60 mL/min (p = 0.002). Markers of inflammation (CRP, fibrinogen, and serum ferritin) have not shown a statistically significant difference between the two groups. These data suggest the greater utility of the assay of NT-proBNP compared with cTnT in patients in the early stages of CKD.
Figure 1. Linear regression plot. LVMI (g/m2) versus eGFR (mL/min). Negative correlation was found between the two parameters (r = −0.489, p < 0.001).
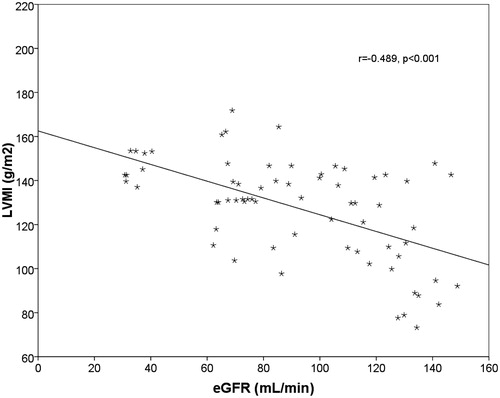
Figure 2. Linear regression plot. LVMI (g/m2) versus NT-proBNP (pg/dL). Positive correlation was found between the two parameters (r = −0.491, p = 0.017).
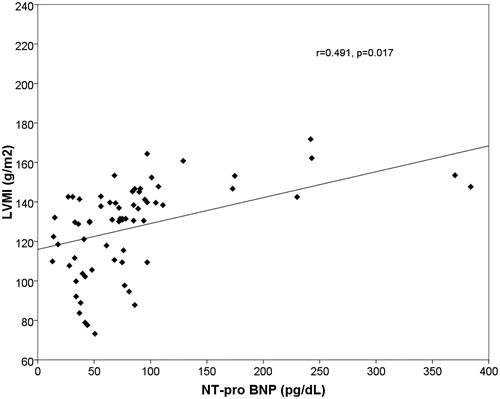
Figure 3. Bar charts with error bars. NT-proBNP (pg/dL) was significantly greater in eGFR 30–89 group (stages 2–3 KDOQI) compared to the control group. Boxes represent means; error bars indicate standard deviation (264.2 ± 155.1 vs. 109.2 ± 56.3, p < 0.001).
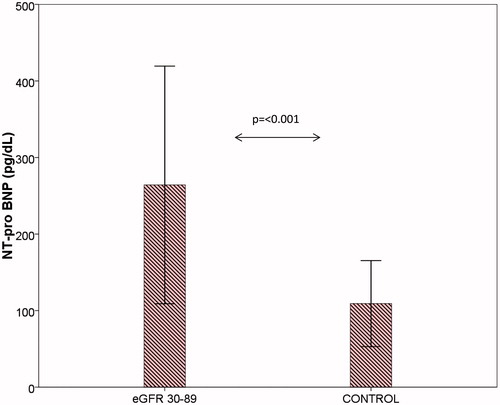
Figure 4. Bar charts with error bars. IMT (+%) was significantly greater in eGFR 30–89 group (stages 2–3 KDOQI) compared to the control group (*p < 0.001, 1.7 ± 0.9 vs. 0.7 ± 0.1), FMD (mm) was significantly lower (**p = 0.048, 15.1 ± 9.1 vs. 19.9 ± 7.2). Boxes represent means; error bars indicate standard deviation.
Discussion
In this study, NT-proBNP and cTnT were evaluated as cardiac biomarkers and found to be associated with nontraditional cardiovascular risk factors such as serum uric acid, homocysteine, and noninvasive predictors of atherosclerosis measured by cIMT, baFMD, and LVMI. NT-proBNP and serum cTnT appear to measure the different aspects of CVDs.Citation28–31 Satyan and othersCitation13 showed an independent correlation of NT-proBNP with left ventricular systolic dysfunction and of cTnT with LVMI in an HD population, a study by WangCitation32 demonstrated that NT-proBNP had the highest diagnostic value for severe LVH and systolic dysfunction compared with cTnT and CRP in PD patients. Experimental models have shown that hypoxic myocardial tissue results in the production of extra BNP, suggesting that BNP could potentially serve as a biomarker of myocardial ischemia.Citation13–15,Citation28,Citation33 These experimental data were confirmed in a number of small-scale clinical studies, which were summarized in a meta-analysis.Citation13–15 These data indicate that BNP is not only linked to LVH and heart failure but that there is a possible role for BNP also in the diagnosis and management of myocardial necrosis. Although cTnT elevation is assumed to be the result of clinically silent myocardial ischemia and micro-infarctions, cTnT also has been found to be elevated in patients with heart failure in the absence of acute ischemia and in CKD patients with LVH without myocardial necrosis.Citation13,Citation14 Furthermore, studies evaluating the relation between cTnT and left ventricular parameters have shown contrasting results. Mallamaci and othersCitation34–36 suggested that cTnT had a significant correlation with LVH and left ventricular systolic dysfunction in 258 HD patients. De Filippi and othersCitation37 found no significant association between cTnT quartiles and LVH in HD patients. Although the accuracy of cardiac biomarkers in evaluating CVDs is improving, the relation between cardiac biomarkers and anatomical and functional assessment of atherosclerosis in CKD remains unclear.Citation14 Experimental modelsCitation13,Citation28 have shown that even a modest resection of renal parenchyma causes LVH, microvessel disease, and cardiac fibrosis with wall thickening of intramyocardial arterioles in the hypertrophied heart. Our data suggest the greater utility of the assay of NT-proBNP compared with cTnT in patients in the early stages of CKD. Also, inflammation is one of the pathogenetic events responsible for CVDs in CKD.Citation29 Therefore, there is a possibility that the observed association between increased NT-proBNP and LVH could be mediated by chronic inflammation, but in this study, the markers of inflammation (CRP, fibrinogen, and serum ferritin) have not shown a statistically significant difference between patients in the stage 2/3 KDOQI and healthy controls. These data could indicate that chronic inflammation independently affect CV mortality in the CKD population or in more advanced disease stages. Several studies have confirmed the association between homocysteine and serum uric acid levels with endothelial dysfunction and atherosclerosis in various patient populations;Citation6–8 indeed, hyperuricemia and hyperhomocysteinemia are highly prevalent in CKD and may have a role as uremic toxins in these patients. Hyperuricemia is a potentially modifiable cardiovascular risk factor and might play a significant role in endothelial dysfunction, inflammation, and atherosclerosis; indeed in different randomized controlled trials, allopurinol treatment resulted in the improvement of oxidative stress, endothelial function, and progression of kidney disease.Citation9–11 The complex interaction between hyperhomocysteinemia, oxidative stress, and microinflammation may result in accelerated and early atherosclerosis in CKD patients.Citation7,Citation30 Several mechanisms for hyperhomocysteinemia-associated vascular disease have been proposed, including potentiation of atherosclerosis through endothelial dysfunction, smooth muscle cell hyperplasia, and increased production of oxidized lipids.Citation6,Citation8,Citation31 Previous carotid B-mode ultrasound studies suggested that hyperhomocysteinemia may play a role in the progression of atherosclerosis and that elevated plasma homocysteine level in CKD is associated with the increase of cIMT. Ultrasonographic evaluation of cIMT is a valid and reproducible indicator of atherosclerotic disease.Citation38,Citation39 An increased cIMT in patients with cardiovascular risk factors is associated with systemic atherosclerosis, representing as an independent predictor factor of cardiovascular/cerebrovascular events. Cardiovascular risk factors promote the development of endothelial dysfunction, characterized by the impairment of endothelium-dependent vasodilatation and procoagulant/proinflammatory endothelial activities. NO is the principal mediator of FMD generated from the activation of endothelial nitric oxide synthetase (eNOS) present in the endothelium; thus, the FMD can be detected only in the presence of integral endothelium.Citation31 The association between cIMT, baFMD, and LVH may suggest that the markers for the development of atherosclerotic disease can be a powerful noninvasive, reproducible, inexpensive and well-tolerated tool to assess CV risk in CKD patients.Citation31,Citation38,Citation39
A limitation of our study was the relatively small sample size.
Conclusion
In conclusion, these data indicate that a finding of an increased cTnT or NT-proBNP in subjects with CKD stages 2/3 should be taken seriously as a prognostic marker for a worse cardiovascular outcome and not be discarded as purely a reflection of reduced renal clearance. Serum NT-proBNP appeared to be a useful clinical biomarker for evaluating noninvasive predictors of atherosclerosis already in stage 2 KDOQI, whereas serum cTnT levels seem to be a more useful marker in more advanced disease stages (stage 3 KDOQI).
In CKD patients, there should be a careful evaluation of the serum cardiac biomarkers, nontraditional risk factors, and noninvasive predictors of atherosclerosis already in stage 2/3 KDOQI.
Declaration of interest
The authors report no conflicts of interest. The manuscript has been seen and approved by all authors. This study was not funded. The manuscript is not under consideration for publication elsewhere.
References
- Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42(5):1050–1065
- Kasiske BL, Chavers B, Foley R, et al. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl. 1):S1–S266
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5):S112–S119
- Muntner P, He J, Hamm L, Loria C, Whelton PK. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002;13(3):745–753
- Lai S, Coppola B, Dimko M, et al. Vitamin D deficiency, insulin resistance, and ventricular hypertrophy in the early stages of chronic kidney disease. Ren Fail. 2014;36(1):58–64
- Perna AF, Castaldo P, Ingrosso D, De Santo NG. Homocysteine, a new cardiovascular risk factor, is also a powerful uremic toxin. J Nephrol. 1999;12:230–240
- Perna AF, Capasso R, Acanfora F, et al. Toxic effects of hyperhomocysteinemia in chronic renal failure and in uremia: cardiovascular and metabolic consequences. Semin Nephrol. 2006;26(1):20–23
- Wierzbicki AS. Homocysteine and cardiovascular disease: A review of the evidence. Diabetes Vasc Dis Res 2007;4(2):143–150
- Navaneethan SD, Beddhu S. Associations of serum uric acid with cardiovascular events and mortality in moderate chronic kidney disease. Nephrol Dial Transplant. 2009;24:1260–1266
- Madero M, Sarnak MJ, Menon V. Uric acid and long-term outcomes in CKD. Am J Kidney Dis. 2009; 53(5):796–803
- Lai S, Mariotti A, Coppola B, et al. Uricemia and homocysteinemia: nontraditional risk factors in the early stages of chronic kidney disease-preliminary data. Eur Rev Med Pharmacol Sci. 2014;18(7):1010–1017
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New Engl J Med. 2004;351(13):1296–1305
- Satyan S, Light RP, Agarwal R. Relationships of N-terminal pro-B-natriuretic peptide and cardiac troponin T to left ventricular mass and function and mortality in asymptomatic hemodialysis patients. Am J Kidney Dis. 2007;50(6):1009–1019
- Caliskan Y, Ozkok A, Akagun T, et al. Cardiac biomarkers and noninvasive predictors of atherosclerosis in chronic peritoneal dialysis patients. Kidney Blood Press Res. 2012;35(5):340–348
- Jafri L, Kashif W, Tai J, et al. B-type natriuretic peptide versus amino terminal pro-B type natriuretic peptide: selecting the optimal heart failure marker in patients with impaired kidney function. BMC Nephrol. 2013;14:117
- Scheven L, de Jong PE, Hillege HL, et al.; PREVEND study group. High-sensitive troponin T and N-terminal pro-B type natriuretic peptide are associated with cardiovascular events despite the cross-sectional association with albuminuria and glomerular filtration rate. Eur Heart J. 2012;33(18):2272–2281
- Tagore R, Ling LH, Yang H, et al. Natriuretic peptides in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3(6):1644–1651
- Khan IA, Fink J, Nass C, et al. N-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide for identifying coronary artery disease and left ventricular hypertrophyin ambulatory chronic kidney disease patients. Am J Cardiol. 2006;97(10):1530–1534
- Kocyigit I, Gungor O, Unal A, et al. The effect of strict volume control on cardiac biomarker and arterial stiffness in peritoneal dialysis patients. Clin Nephrol. 2014;81(4):238–246
- Stenvinkel P, Carrero JJ, Axelsson J, et al. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol. 2008;3(2):505–521
- Williams B, Poulter NR, Brown MJ, et al.; BHS guidelines working party, for the British Hypertension Society. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): Summary. BMJ. 2004;328(7440):634–640
- Ho CY, Solomon SD. A clinician's guide to tissue Doppler imaging. Circulation. 2006;113(10):e396–e398
- Patel S, Celermajer DS. Assessment of vascular disease using arterial flow mediated dilatation. Pharmacol Rep. 2006;58(Suppl):3–7
- Corretti MC, Anderson TJ, Benjamin EJ. Guidelines for the ultrasound assessment of endothelial-dependent flow mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265
- Lang RM, Bierig M, Devereux RB, et al.; Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463
- Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367
- Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55(4):613–618
- Amann K, Neimeier KA, Schwarz U, et al. Rats with moderate renal failure show capillary deficit in heart but not skeletal muscle. Am J Kidney Dis. 1997;30:382–388
- Desai AS1, Toto R, Jarolim P, et al. Association between cardiac biomarkers and the development of ESRD in patients with type 2 diabetes mellitus, anemia, and CKD. Am J Kidney Dis. 2011;58(5):717–728
- Nanayakkara PW, Kiefte-de Jong JC, Stehouwer CD, et al. Association between global leukocyte DNA methylation, renal function, carotid intima-media thickness and plasma homocysteine in patients with stage 2–4 chronic kidney disease. Nephrol Dial Transplant. 2008;23(8):2586–2592
- Yeboah J, Burke GL, Crouse JR, et al. Relationship between brachial flow-mediated dilation and carotid intima-media thickness in an elderly cohort: The Cardiovascular Health Study. Atherosclerosis. 2008;197(2):840–845
- Wang AY. Clinical utility of natriuretic peptides in dialysis patients. Semin Dial. 2012;25(3):326–333
- Horii M1, Matsumoto T, Uemura S, et al. Prognostic value of B-type natriuretic peptide and its amino-terminal proBNP fragment for cardiovascular events with stratification by renal function. J Cardiol. 2013;61(6):410–416
- Mallamaci F, Zoccali C, Parlongo S, et al. Troponin is related to left ventricular mass and predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2002;40:68–75
- Mallamaci F, Zoccali C, Parlongo S, et al. Diagnostic value of troponin T for alterations in left ventricular mass and function in dialysis patients. Kidney Int. 2002;62:1884–1890
- Mallamaci F, Tripepi G. Value of troponin T as a screening test for left ventricular hypertrophy in CKD. Am J Kidney Dis. 2013;61(5):689–691
- De Filippi C, Wasserman S, Rosanio S, et al. Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA. 2003;290:353–359
- Campuzano R, Moya JL, García-Lledó A, et al. Endothelial dysfunction, intima-media thickness and coronary reserve in relation to risk factors and Framingham score in patients without clinical atherosclerosis. J Hypertens. 2006;24(8):1581–1588
- Yan RT, Anderson TJ, Charbonneau F, Title L, Verma S, Lonn E. Relationship between carotid artery intima-media thickness and brachial arteryflow-mediated dilation in middle-aged healthy men. J Am Coll Cardiol. 2005;45(12):1980–1986
