Abstract
Background and aim: There is an increased mortality risk in long-term hemodialysis patients of renal failure due to the chronic inflammation. The relationship between the chronic renal failure (CRF) and the role of familial genetic markers remains incompletely understood. In the current study, it was aimed to find out the prevalence of common MEFV gene mutations and BcII polymorphism in serum amyloid A1 (SAA1) gene in chronic renal patients (CRF) who require long-term hemodialysis. Method: Current cohort includes 242 CRF patients and 245 healthy individuals from the same population. Total genomic DNA was isolated from peripheral blood–EDTA samples and genotyping of target MEFV gene was carried out by reverse hybridization Strip Assay and real-time techniques. The SAA1 gene was genotyped by the BclI-RFLP method. Results: Increased mutated MEFV genotypes were found in current CRF patients when compared with the control group from the same ethnicity and the difference was statistically significant (Table 2) (OR: 4.9401, 95% CI: 3.0694–7.9509), p < 0.0001. The most frequent point mutations were M694V and E148Q. The mutated T allel frequency in the SAA1 gene was also different when compared with the healthy controls and the difference was found to be statistically significant (χ2: 13.18; p = 0.000). Conclusions: The current results indicate the germ-line mutations in both genetic biomarkers (MEFV and SAA1 genes) that are related to inflammation and amyloidosis processes may play a crucial role in CRF pathogenesis due to the long-term chronic inflammation.
Introduction
Chronic renal failure (CRF) is an important global health problem that affects approximately 10% of the world population.Citation1 In Turkey, the large portion of patients (7.5 million) is suffering from chronic kidney disease (CKD) and 60,000 of those patients are diagnosed as CRF.Citation2 In some complicated cases, the CKD is usually has irreversible treating complications and commonly progress to end-stage renal disease (ESRD).Citation3,Citation4 Genetics and non-genetics parameters were reported in the pathogenesis of CKD and CRF. The common clinical demographics for CRF are obesity, untreated diabetes, hypertensive nephropathy, renovascular diseases, obstructive uropathy, primary glomerulonephritis, and amyloidosis.Citation4–6 CRF is also associated with uremic toxins, oxidative stress, inflammation, low folate status, and increased plasma levels of homocysteine.Citation5,Citation6 Literature findings showed that genetic parameters such as chromosomal rearrangements, point mutations, gene polymorphisms, environmental factors, lifestyles, and epigenetic alterations may also cause the CRF.Citation7–9 Amyloidosis is a major complication for chronic inflammation diseases that ends by heavy proteinuria, progressive renal deterioration, and leads to ESRD.Citation10 Renal damage may result from the extracellular deposition of proteolytic fragments of the acute-phase reactant SAA as amyloid fibrils. Amyloidosis is an important parameter for CRF that is characterized by aggregation of serum amyloid A protein (SAA) into amyloid fibrils and deposition in extracellular tissues such as kidney, liver, spleen, heart, and testes that lead to CRF.Citation11–14 Recent studies show that FMF and SAA1:1 genotype are associated with amyloidosis.Citation15 In the majority of people, acute phase AA amyloid proteins are encoded by polymorphic SAA1 gene that shows variation in different ethnic groups.Citation13,Citation14 Two single nucleotide polymorphisms were reported in exon 3 (codon 2995 C < T and codon 3010 C > T) of SAA1 gene.Citation16 The codon 3010 C > T polymorphism has been reported to be associated with increased risk of AA amyloidosis in Japanese and Caucasian populations.Citation17–19 Some researchers have claimed that SAA synthesis prevents amyloid deposition and regressed clinical outcomes in the patients with amyloidosis during inflammation processes.Citation20,Citation21
FMF is a periodic fever disease that especially occurs in Jews, Arabic, Armenian, and Turks. Disease resulted by variable point mutations in MEFV gene is located on human chromosome 16. MEFV gene encodes pyrin protein which expressed by myeloid cells that have an arrester role on inflammatory disease.Citation22 FMF affects multi-organ including kidney as the major target organ with some clinical findings such as recurrent painful self-limited febrile attacks including abdominal, articular and chest pain, inflammation of serosal cavities, erysipelas like erythema, and myalgia.Citation22,Citation23 The M694V, E148Q, M680I, and V726A are common point mutations that are located on exons 2, 3, 5, and 10 which are playing crucial role in FMF pathogenesis. It has been reported that homozygously mutated patients for M694V have a seven-fold increase risk in the incidence of renal amyloidosis when compared with the non-mutated individuals.Citation24,Citation25 The recent literature findings showed that there is a strong correlation between amyloidosis based on FMF and CRF pathogeneses but the relationship between chronic kidney failure and role of germ-line genetic markers remains incompletely understood.
In the current study, we aimed to investigate the mutation profiles for MEFV and serum amyloid A1 (SAA1) genes in CRF patients which require long-term hemodialysis in Turkish population.
Materials and methods
Study population
Two hundred and forty-two Turkish patients who suffered from CRF and required long term hemodialysis in the five different dialysis centers from Sivas, Turkey, were enrolled in the current study. The results were compared with the 245 healthy individuals of same ethnicity without CRF and familial renal disease history. All applications were approved by the local ethics committee of Cumhuriyet University (Ref. no. 2009-CUBAP/T361) and informed consent was obtained from all the subjects.
Target MEFV genotyping
Peripheral blood–EDTA samples were used for the genomic DNA isolation and DNA was isolated by the spin-colon method (Roche, Mannheim, Germany) in the current project. Common 12 mutation profiles for MEFV gene (E148Q, P369S, F479L, M680I (G/C), M680I (G/A), 1692del, M694V, M694I, K695R, V726A, A744S, and R761H) were genotyped by strip assay reverse hybridization technique (Vienna Lab, Vienna, Austria) for the current CRF cohort and healthy individuals. Some suspicious samples were also genotyped for exons 2 and 10 for the target MEFV gene by direct Sanger sequencing technique in the presented results. PCR products were purified and the target DNA sequences were analyzed on an ABI Prism Genetic Analyser 310 (Applied Biosystems, Foster City, CA).
Target SAA1 genotyping
The PCR-RFLP technique was used for serum amyloid A1 gene (SAA1) profiling. The 518 bps of exon 3 for target SAA1 gene was amplified and genotyped by using BcII restriction endonuclease enzyme.
The sequences of primers were the following: F: 5′-GCCAATTACATCGGCTCAG-3′ and R: 5′-TGGCCAAAGAATCTCTGGAT-3′.
Amplifications of exon 3 of SAA1 gene were performed in a volume of 50 µl reaction mixtures containing 200 µM dNTPs (MBI, Fermentas, Amherst, NY), 0.5 µM primers, 30 ng template DNA, 10 × Taq polymerase buffer, and 1.5 U µl Taq polymerase (Boehringer, Mannheim). After pre-denaturation of the mixture at 95 °C for 5 min, the target gene was amplified with 35 cycles in Amplitron I DNA Thermal Cycler (Thermolyne, Dubuque, IA) under the following conditions: denaturation at 95 °C for 12 min, annealing at 60 °C for 1 min, extension at 72 °C for 30 s, and 72 °C 5 min for post-extension. One microliter of PCR products (518 bps) were used for cognate enzyme digestion. Digested (498 bps and 20 bps) and non-digested fragments were identified on 1.5% agarose gel electrophoresis.
Statistical analysis
Statistical analysis was performed by using SPSS version 16 (SPSS, Chicago, IL) for comparing the current results. The homozygous, heterozygous mutation frequencies, and polymorphic alleles for the target genes in CRF and control groups were also compared by using Chi-square analysis. The p < 0.05 values were considered as statistically significant and significant mutation profiles were discussed in the current report.
Results
Presented results show germ-line mutations in MEFV and RFLP profiles for SAA1 genes in CRF patients and healthy controls. Peripheral blood–EDTA samples from healthy controls and CRF patients were used for genotyping in the current study. The cohort was composed of 242 CRF patients [(123 males (50.8%) and 119 females (49.2%)] of mean age 57.02 ± 71.4 (18–75) who were compared with the 212 healthy individuals [(152 males (62.0%) and 93 females (38.0%)] of mean age 57.24 ± 9.71 (32–68) from the same population. Some clinical characteristics such as hypertension, diabetes, atherosclerosis, nephrolithiasis, and polycystic kidney were found in 127/52.5%, 65/26.9%, 33/13.6%, 17/7.0%, and 15/6.2% for the current CRF cohort, respectively (). Twenty-eight patients (11.2%) have parental consanguinity and six patients (2.5%) have familial FMF history in the current CRF cohort ().
Table 1. The prevalence of some clinical characteristics such as mean age, gender, parental consanquinity, and HCV, HBV status of the current CRF cohort.
MEFV mutations were studied by the multiplex PCR-based reverse hybridization Strip Assay method and some suspicious cases were analyzed by direct Sanger sequencing technique. The PCR–RFLP technique was used for serum amyloid A1 gene (SAA1) profiling in the presented results. Homozygous and/or heterozygous MEFV gene point mutations were detected in the current CRF cohort. No mutations were detected in 149 (61.6%) CRF patients but one and/or compound mutations were detected in the current 93 (38.4%) CRF patients ( and ). Twenty-seven (11%) randomly selected control individuals from the same population have various mutations and the rest (89%) have showed wild profile for target MEFV gene. The mutation prevalence for MEFV gene was statistically significant when comparing both groups (p < 0.0001, OR: 4.940, 95% CI: 3.0694–7.9509) (). Fifty-four CRF patients (22.3%) were mutated for M694V [46 (19%) heterozygous and 2 (3.3%) homozygous)], 25 patients (10.4%) were mutated for E148Q [24 (9.9%) heterozygous and 1 (0.4%) homozygous)], six patients (2.48%) were mutated for V726A [4 (1.6%) heterozygous and 2 (0.8%) homozygous)], seven patients (2.9%) were heterozygously mutated for P369S, one patient (0.4%) was heterozygously mutated for codon F476L, five patients (2%) were heterozygous for M680I G/C, two patients (0.8%) were heterozygously mutated for codon K695R, and one patient (0.4%) was heterozygously mutated for codon A744S (). The current results of 242 CRF patients revealed that M694V was the most frequent mutation (19%), followed by E148Q (9%), V726A (2.48%), and M680I (G/C) (2%) in the presented CRF cohort. Increased mutated MEFV genotypes were found in current CRF patients compared with the control group from the same population and the difference was statistically significant () (p < 0.0001, OR: 4.9401, 95% CI: 3.0694–7.9509).
Figure 1. Sanger sequence profile shows the heterozygous A/G transition point mutation in codon M694V of exon 10 in MEFV gene (arrow) in one of the CRF cases that require long-term hemodialysis.
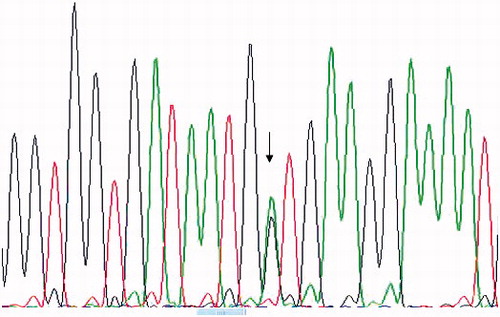
Table 2. Shows prevalence and mutation types that detected in the current CRF patients and control group.
The gene polymorphism for SAA1 exon 3 (3010, C/T) was determined by BclI-RFLP technique for CRF patients and results were also compared with the healthy controls from the same ethnicity. The CC genotypes for SAA1 gene were 38%, the CT genotypes for SAA1 gene were 32%, and the TT genotypes for SAA1 gene were 30% for the current CRF cohort. The SAA1 genotypes showed 46%, 33%, and 21% for the current healthy controls (). The mutated T allel frequency was 0.455 for CRF patients and 0.375 for healthy controls. The difference was found to be statistically significant (χ2: 13.18, p = 0.000; ).
Table 3. The genotype and allele frequency of C/T polymorphism of SAA1 gene exon 3 in control and CRF patients after BclI-RFLP-based profiling.
Discussions
Chronic kidney disorders exhibit a monogenic, polygenic multifactorial, and epigenetic patterns of inheritance.Citation26 Recent literature findings on genetic studies have identified many predictive genetic markers that are associated with an increased risk of developing CRF.Citation26–28 Inflammation processes are not only responsible for the etiology of CRF but may also play an important role in cardiovascular diseases and CRF. Strong association was reported between complement receptor 1 gene and end-stage renal disease (ESRD).Citation29 Zhou and YinCitation30 have claimed that the eNOS Glu298Asp gene polymorphism is associated with the ESRD risk. Kai et al.Citation31 have claimed that germ-line mutations based on thrombophilic disorders such as diabetic nephropathy, polycystic renal disease, hypertension, and thrombophilia with renal failure are possible mechanisms explaining the implication of the thrombophilic states in kidney allograft thrombosis and renal rejection.
The high level of serum concentration of SAA reflects the activity of chronic inflammation and it is a prerequisite for developing AA amyloidosis. In the present study, we investigated possible associations between germ-line mutations of MEFV and RFLP profiles for polymorphic SAA1 C3010T SNP in CRF patients and healthy controls from the same ethnicity. Clinical demographics of hypertension, diabetes, atherosclerosis, nephrolithiasis, and polycystic kidney were found in 127/52.5%, 65/26.9%, 33/13.6%, 17/7.0%, and 15/6.2% in the presented CRF cohort, respectively. Current results of 242 CRF patients revealed that M694V was the most frequent mutation (19%), followed by E148Q (9%), V726A (2.48%), and M680I (G/C) (2%), in the presented CRF cohort. The mutation prevalence for MEFV gene was statistically significant when comparing CRF and healthy individuals (p < 0.0001, OR: 4.9401, 95% CI: 3.0694–7.9509). The mutated T allel frequency was 0.455 for CRF patients and 0.375 for healthy controls for SAA1 3010C > T SNP marker. That difference was also found to be statistically significant (χ2: 13.18, p = 0.000). Briefly, increased M694V and E148Q point mutations in MEFV and T allele frequency in SAA1 3010C > T SNP were detected in the current CRF cohort. In the present study, a significant association was found in mutated MEFV and T alleles in SAA1 gene profiles in CRF patients when compared with the control group. Some researchers have claimed that the combined effect of homozygous mutated M694V and genotype alpha/alpha of SAA1 gene is associated with a seven-fold increase in the incidence of renal amyloidosis.Citation32–34 Genotype–phenotype studies tried to confirm the association between specific point mutations for some amyloidosis-based target genes and CRF.Citation34 The present study associates CRF with specific MEFV gene mutations. Moreover, it also associates SAA1 3010C > T SNP in CRF patients with some clinical demographics. This suggests that in addition to being a marker for high risk for AA amyloidosis, SAA1 SNP may also be useful as a risk factor for CRF pathogenesis and further studies are needed to confirm that association.
In conclusion, the current preliminary results suggest that germ-line genetic polymorphisms located in MEFV and SAA1 genes related to inflammation and amyloidosis processes could help to predict risk of developing CRF pathogenesis due to the long-term chronic inflammation. Results are needed to be confirmed by a large scale of sample size from different ethnic groups for the advance understanding of molecular triggers of disease.
Acknowledgements
The current results are dedicated to Dr. Dursun Inan, who is one of the co-authors in the current project, who died in a worst avalanche accident in the date of 25th January 2009 (Zigana, Turkey). Authors also thank Sivas Governor Nevzat Dalmaz for his valuable support to our project
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. This project was funded by CUBAP, Cumhuriyet University, Sivas, Turkey.
References
- Palmer SC, Vecchio M, Craig JC, et al. Association between depression and death in people with CKD: A meta-analysis of cohort studies. Am J Kidney Dis. 2013;62(3):493–505
- Altiparmak MR, Seyahi N, Trabulus S, et al. Applicability of a different estimation equation of glomerular filtration rate in Turkey. Ren Fail. 2013;35(8):1116–1123
- Lewis R. An overview of chronic kidney disease in older people. Nurs Older People. 2013;25(10):31–38
- Chandna S, Da Silva-Gane M, Marshall C, Warwicker P, Greenwood RN, Farrington K. Survival of elderly patients with stage 5CKD: Comparison of conservative management and renal replacement therapy. Nephrol Dial Transpl. 2001;26(5):1608–1614
- Satta E, Perna AF, Lombardi C, et al. Hyperhomocysteinemia in chronic renal failure. G Ital Nefrol. 2006;23(5):480–489
- Miao XH, Wang CG, Hu BQ, Li A, Chen CB, Song WQ. TGF-beta1 immunohistochemistry and promoter methylation in chronic renal failure rats treated with Uremic Clearance Granules. Folia Histochem Cytobiol. 2010;48(2):284–291
- Whyte MP, Leelawattana R, Reinus WR, Yang C, Mumm S, Novack DV. Acute severe hypercalcemia after traumatic fractures and immobilization in hypophosphatasia complicated by chronic renal failure. J Clin Endocrinol Metab. 2013;98(12):4606–4612
- Deltas C, Pierides A, Voskarides K. Molecular genetics of familial hematuric diseases. Nephrol Dial Transplant. 2013;28(12):2946–2960
- Aydin B, Ipek MS, Ozaltin F, et al. A novel mutation of laminin β-2 gene in Pierson syndrome manifested with nephrotic syndrome in the early neonatal period. Genet Couns. 2013;24(2):141–147
- Ben-Chetrit E, Backenroth R. Amyloidosis induced, end stage renal disease in patients with familial Mediterranean fever is highly associated with point mutations in the MEFV gene. Ann Rheum Dis. 2001;60(2):146–149
- Dember LM. Amyloidosis-associated kidney disease. J Am Soc Nephrol. 2006;17:3458–3471
- Eirin A, Irazabal MV, Gertz MA, et al. Clinical features of patients with immunoglobulin light chain amyloidosis (AL) with vascularlimited deposition in the kidney. Nephrol Dial Transplant. 2012;27:1097–1101
- Cerquaglia C, Diaco M, Nucera G, La Regina M, Montalto M, Manna R. Pharmacological and clinical basis of treatment of familial Mediterranean fever (FMF) with colchicine or analogues: An update. Curr Drug Targets Inflam Allergy. 2005;4:117–124
- Tang W, McDonald SP, Hawley CM, et al. End-stage renal failure due to amyloidosis: Outcomes in 490 ANZDATA registry cases. Nephrol Dial Transplant. 2013;28(2):455–461
- Delibaş A, Oner A, Balci B, et al. Genetic risk factors of amyloidogenesis in familial Mediterranean fever. Am J Nephrol. 2005;25(5):434–440
- Ajiro J, Narita I, Sato F, et al. SAA1 gene polymorphisms and the risk of AA amyloidosis in Japanese patients with rheumatoid arthritis. Mod Rheumatol. 2006;16(5):294–299
- Baba S, Masago SA, Takahashi T, et al. A novel allelic variant of serum amyloid A, SAA1 gamma: Genomic evidence, evolution, frequency, and implication as a risk factor for reactive systemic AA-amyloidosis. Hum Mol Genet. 1995;4(6):1083–1087
- Moriguchi M, Terai C, Koseki Y, et al. Influence of genotypes at SAA1 and SAA2 loci on the development and the length of latent period of secondary AA-amyloidosis in patients with rheumatoid arthritis. Hum Genet. 1999;105(4):360–366
- Booth DR, Booth SE, Gillmore JD, Hawkins PN, Pepys MB. SAA1 alleles as risk factors in reactive systemic AA amyloidosis. Amyloid. 1998;5(4):262–265
- Lachmann HJ, Goodman HJ, Gilbertson JA, et al. Natural history and outcome in systemic AA amyloidosis. N Engl J Med. 2007;356(23):2361–2371
- Simons JP, Al-Shawi R, Ellmerich S, et al. Pathogenetic mechanisms of amyloid A amyloidosis. Proc Natl Acad Sci USA. 2013;110(40):16115–16120
- Gershoni-Baruch R, Brik R, Zacks N, Shinawi M, Lidar M, Livneh A. The contribution of genotypes at the MEFV and SAA1 loci to amyloidosis and disease severity in patients with familial Mediterranean fever. Arthritis Rheum. 2003;48:1149–1155
- Grateau G. The relation between familial Mediterranean fever and amyloidosis. Curr Opin Rheumatol. 2000;12(1):61–64
- Ozdemir O, Sezgin I, Kurtulgan KH, et al. Prevalence of known mutations in the MEFV gene in a population screening with high rate of carriers. Mol Biol Rep. 2011;38(5):3195–3200
- Cazeneuve C, Ajrapetyan H, Papin S, et al. Identification of MEFV-independent modifying genetic factors for familial Mediterranean fever. Am J Hum Genet. 2000;67:1136–1143
- Hallan SI, Orth SR. Smoking is a risk factor in the progression to kidney failure. Kidney Int. 2011;80(5):516–523
- Sezgin I, Koksal B, Bagci G, Kurtulgan HK, Ozdemir O. CCR2 polymorphism in chronic renal failure patients requiring long-term hemodialysis. Intern Med. 2011;50:2457–2461
- De Serres SA, Varghese JC, Levin A. Biomarkers in native and transplant kidneys: Opportunities to improve prediction of outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens. 2012;21(6):619–627
- Buraczynska M, Ksiazek P, Wacinski P, Zukowski P, Dragan M, Bednarek-Skublewska A. Complement receptor 1 gene polymorphism and cardiovascular disease in dialyzed end-stage renal disease patients. Hum Immunol. 2010;71(9):878–882
- Zhou TB, Yin SS. Association of endothelial nitric oxide synthase Glu298Asp gene polymorphism with the risk of end-stage renal disease. Ren Fail. 2013;35(4):573–578
- Klai S, Fekih-Mrissa N, Ghachem A, et al. Thrombophilic disorders: A real threat to patients with end-stage renal disease on hemodialysis and at the time of renal transplantation. Blood Coagul Fibrinol. 2012;23(5):406–410
- Medlej-Hashim M, Delague V, Chouery E, et al. Amyloidosis in familial Mediterranean fever patients: Correlation with MEFV genotype and SAA1 and MICA polymorphisms effects. BMC Med Genet. 2004;5(4):1–6
- Cazeneuve C, Ajrapetyan H, Papin S, et al. Identification of MEFV-independent modifying genetic factors for familial Mediterranean fever. Am J Hum Genet. 2000;67(5):1136–1143
- Gershoni-Baruch R, Brik R, Zacks N, Shinawi M, Lidar M, Livneh A. The contribution of genotypes at the MEFV and SAA1 loci to amyloidosis and disease severity in patients with familial Mediterranean fever. Arthritis Rheum. 2003;48(4):1149–1155
