Abstract
Background: The aim of this study was to investigate the effects of exposure to a 900-MHz electromagnetic field (EMF) produced by mobile phones on the renal development of prenatal rats. Histopathological changes and apoptosis in the kidneys, together with levels of urea, creatinine and electrolyte in serum were determined. Methods: A total of 14 Sprague–Dawley rats were studied. Pregnant rats were divided into two equal groups: a control group and an EMF-exposed group. The study group was exposed to 900-MHz of EMF during the first 20 days of pregnancy, while the control group was unexposed to EMF. Sections obtained from paraffin blocks were stained for caspase-3 by immunohistochemistry, hematoxylin–eosin and Masson’s trichrome. Results: Mild congestion and tubular defects, and dilatation of Bowman’s capsule were observed in the kidney tissues of rats in the exposed group. Apoptosis was evaluated using anti-caspase-3; stronger positive staining was observed in the renal tubular cells in the study group than those of the control group. Although there was a significant difference between the study and control groups in terms of K+ level (p < 0.05), no significant difference was observed in the other parameters studied (p > 0.05). Conclusion: Our study shows that the electromagnetic waves propagated from mobile phones have harmful effects on the renal development of prenatal rats.
Introduction
Many electrical devices, including cell phones, produce electromagnetic radiation (EMR). As a result, people are continuously exposed to these magnetic waves. Cell phone use has increased worldwide in recent years (>80%). EMR from excessive mobile phone usage may pose a threat to human health. Research on possible fetal harm caused by EMR during pregnancy has revealed contradicting results.Citation1–4 However, there are some postnatal studies that established a correlation with cancer development, cellular anti-proliferative effects and neurological disorders due to radiation produced from mobile phones or base stations.Citation5,Citation6
Caspases play important roles during apoptosis, inflammation and proliferation.Citation7 Caspase-3 activity depends on cytological Ca+2 concentration. Ca+2 is the main ion in the development of biological processes such as hormone secretion and mitotic division.Citation8 Ca+2 levels can also facilitate apoptotic cell deaths in pathological cases such as necrosis.Citation9 Increase in Ca+2 concentrations initiates the early and late apoptotic pathways.Citation10 In this study, we investigated the histological alterations, and presence of apoptosis in the kidneys of rats exposed to electromagnetic field (EMF) radiation from mobile phones 24 hours a day during the first 20 days of the prenatal period. In addition, the serum levels of some biochemical compounds (Ca+2, K+, CI−, urea and creatinine) were also determined.
Materials and methods
The study protocol was reviewed and approved by the Local Ethical Committee for Animal Experiments in Recep Tayyip Erdoğan University School of Medicine in Turkey (Approval date: 16 January 2014; meeting number: 2014/6).
Animals and experimental procedures
A total of six pregnant Sprague–Dawley rats weighing 260–290 grams were included in this study. The rats were maintained on a 24-hour light–dark cycle regiment at a temperature of 21–24 °C with 50–55% relative humidity. All animals had free access to food and water. All procedures involving the animals were designed and performed according to the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
One day before radiation exposure, three female rats were placed in each cage to conceive. One male rat was placed in each cage for mating with the three female rats. The following day, the male rats were removed from the cages. After inserting a pipette into the vaginas of the rats, 0.4 mL of physiological saline solution was administered, and vaginal secretions were collected. The samples were examined by the vaginal smear method using light microscopy. A rat was considered to be at day 0 of pregnancy if the microscopic examination showed presence of sperm. Rats were considered pregnant without the need for microscopic examination of the vaginal secretion if the vaginal membrane was observed during vaginal inspection. After the mating day, three pregnant rats were exposed to radiation emitted from a digital signal generator. The generator (Anritsu MG3670 B type, Kanagawa, Japan), which produces 900 MHz radiofrequency radiation, was used in this study to represent human exposure to global systems for mobile communications. The peak power of the digital signal generator was fixed at 2 W during exposure. For the digital signal generator used in this study, the carrier frequency was 900 MHz, the modulation frequency was 217 Hz, the pulse width was 577 µsec and the maximal peak power was 2 W. The study was performed on 14 rats divided into two groups (n = 7), one control and one study. A generator with an external antenna was placed closely and centrally under the cages. For the study group, rats were exposed to the radiation on talking mode 24 hours a day for 20 days. Nothing was applied to the rats in the control group.
After 60 days, the female baby rats (120–130 g) were intraperitoneally administered a combination of 6 mg/kg of 2% xylazine hydrochloride (Rompun) and 75 mg/kg ketamine hydrochloride (Ketalar). Following anesthesia, the animals were terminated by intracardiac perfusion with 4% formaldehyde. The kidneys were excised, and the specimens were prepared for histopathological studies.
Histopathological examination
For light microscopic examination, the kidneys were fixed in a formaldehyde solution. For general morphological assessment, sections approximately 5 µm in thickness were stained with hematoxylin and eosin. Sections were examined using an Olympus BX51 photomicroscope. During microscopic examination, tissue sections were examined for the presence of damage to the Bowman’s capsule, glomeruli and tubules as well as for vascular congestion and inflammatory cell infiltration. Of these three criteria, each was scored as absent (0), minimal (+), moderate (++) or severe (+++). At least five microscopic fields were examined for scoring.Citation11 Each slide was assessed blindly by two separate investigators.
Two histologists and two pathologists blindly graded caspase-3 immunoreactivity levels of the specimens with a three-point scoring system, grading them as weak (+) or 15% of the region, moderate (++) or 30% of the region, or intense (+++) or 55% of the region. Caspase-3 and apoptosis immunoreactivity levels were detected using the streptavidin-biotin peroxidase method. Kidney tissue samples were deparaffinized in xylene and rehydrated in ethanol series. The sections were incubated in 3% hydrogen peroxide for the inhibition of endogenous peroxidase activity. Nonspecific binding sites of antibodies were blocked with normal bovine serum (1–10%). Primary antibodies to caspase-3 (Abcam13847, Abcam, Cambridge, UK) were diluted 1/50 and incubated for 60 minutes. The slides were rinsed, and a biotinylated secondary antibody (Universal LSAB Kit-K0690, Dako, Denmark) was incubated for 30 minutes. The sections were then incubated with streptavidin–horseradish peroxidase (Universal LSAB Kit-K0690). Bound antibodies were identified by staining with 3,3′-diaminobenzidine and washed in phosphate-buffered saline. A hematoxylin counter stain was performed followed by dehydration, clearing and mounting.
Biochemical assays
Blood samples were taken from all rats for biochemical evaluation. The blood samples (10 mL) were collected into tubes. The blood was separated by centrifugation at 3000 rpm for 10 minutes after standing at room temperature for 15 minutes. Levels of sodium (Na+), potassium (K+), chloride (CI−), calcium (Ca+2), urea and creatinine were determined from serum using commercial kits (Architect c 16,000, Autoanalyzer, Abbott Diagnostics, Waltham, MA).
Statistical analysis
According to the Kruskal–Wallis test, there was no statistically significant difference in the histopathological findings between the two groups (p > 0.05; ). Levene’s test for equality of variances was used as the independent-sample test, and a statistically significant difference was observed in K+ values (p < 0.05) between the two groups; however, no significant difference was observed in the other parameters (p > 0.05; ).
Table 1. Comparison of histopathologic findings of control group and study group.
Table 2. Comparison of biochemical parameters of control group and study group.
Results
Histopathological findings
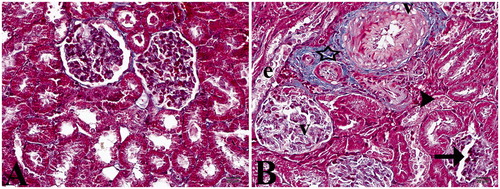
Although no pathological finding was detected by staining with hematoxylin and Masson’s trichrome in the control group, cell structures with normal morphological appearance were observed in the glomerulus and tubular structures ( and ). Histopathological examination of the renal tissue of EMF-exposed rats revealed lower eosinophilic staining than the controls. In addition, mild dilatation of the Bowman’s capsules (60%), tubular lumen dilatation and integration loss and desquamations and mild degenerations due to distention, especially in distal tubule epitheliums, were prominent in this study group. Furthermore, glomerular vacuolization-induced deformations and dilatation were higher by 45% (). Although no fibrosis was detected in the glomerular basal membranes by histochemical staining, a mild increase in connective tissue and vacuolar degeneration was evident in the study group ().
Figure 1. (A) Control group. (B) Study group: (d) tubular dilatation, (v) vacuolization, (e) epithelium desquamation, arrow head; cell degenerations, arrow; dilatation of Bowman’s capsule (Hematoxylin–eosin staining, 40×).
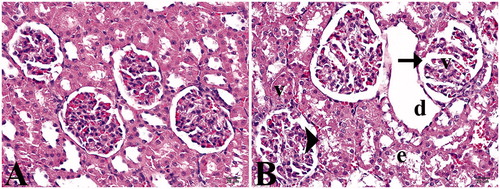
Figure 2. (A) Control group. (B) Study group: star, slightly increase in connective tissue; (v) vacuolization; (e) epithelium desquamation; arrow head, cell degenerations; arrow, dilatation of Bowman’s capsule (Masson’s trichrome staining, 40×).
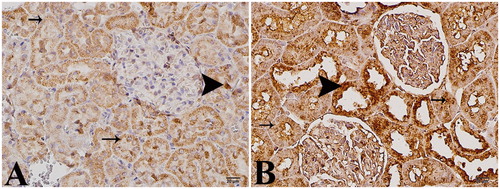
In immunohistochemical examination for apoptosis, mild staining was observed in the control group (). Intense positive staining was observed with caspase-3 in epithelial cells in the distal regions of the parenchyma and tubules of the kidneys in the study group ().
Immunohistochemical findings
There was moderate homogenous staining of the kidney section by caspase-3 in the control group. The staining pattern was granular in the tubular epithelia, which was up to 15% (+) in some cells. Although the glomerular structures were negative, the Bowman’s membranes were graded as 25% (++) positive ().
In the kidney tissues of the study group, there was non-homogenous and moderate immunoreactivity to caspase-3. Proximal tubular epithelium displayed 25% (++) granular-type staining, while distal tubular epithelia showed 20% diffuse staining. There were also mild (+) and moderate (++) staining of the glomerular structures and the Bowman’s membranes, respectively ().
Biochemical findings
In biochemical analyses, a statistically significant difference between K+ levels of the control and study groups was found (p < 0.05). No significant difference was observed for the other components measured (p > 0.05; ).
Discussion
Developments in science and technology have led to the invention of many new devices. These technological developments provide great convenience in the lives of many people; however, they also cause certain adverse effects. Our aim in this study was to investigate the effects of 20 days of exposure to a 900-MHz EMF produced by mobile phones in prenatal rats.
EMF radiation produced by technological devices, including mobile phones, may adversely affect.Citation9,Citation10 EMF, the possible adverse effects of mobile phones on human/fetal health, if any, is still a controversial issue.Citation1
It has been suggested that the EMR produced by mobile phones has adverse effects on human health at the cellular and molecular levels.Citation12 In previous studies, it was reported that EMR produced from mobile phones may affect the immune, nervous, hematological and urinary systems as well as the cardiac functions, genetics and the normal growth and development of humans.Citation12–14 In their study, Ono et al.Citation15 could not determine any significant difference in DNA mutation between the non-exposed group and the embryos exposed to EMR for 16 hours daily for 15 days. However, EMR produced from mobile phones appears to have harmful effects on the human body in two different ways. First, a thermal effect occurs as a result of temperature increase due to absorption of EMR by the body. Second, non-thermal effects such as headache, lack of attention and changes in sleep and brain function are observed.Citation16 Yakymenko and SidorikCitation17 have reported that frequent low-intensity microwave radiation may include over-production of reactive oxygen species, DNA damage, heat shock protein expression and apoptosis. On the other hand, Trosić et al.Citation3 reported no significant difference between the control group and the EMR-exposed animals for DNA damage in the kidney, liver and brain cells of rats.
A number of studies have reported contradictory results on the effects of EMR produced by mobile phones on the reproductive system and fetal development. Dasdag et al.Citation18 found no decrease in sperm count or any abnormal sperm formation in EMR-exposed rats. However, mild histopathological changes have been observed in the testicles of EMF-exposed rats. In his study, PaurlisCitation19 reported that exposure to EMR produced by mobile phones has no strong effect on the development and reproduction of animals. Hancı et al.Citation1 exposed the testicles of male prenatal rats to 900 MHz EMF for 21 days. In this study, they observed a decrease in germ cells in the lumen of seminiferous tubules in the testicles and irregularity and thickening in the basal membranes of seminiferous tubules. Tumkaya et al.Citation2 investigated whether radiation produced by mobile phones has any harmful effect on the testicles of rats in the pubertal period. No harmful effects were detected in their study. Akdag et al.Citation20 reported that long-term exposure to mobile phone radiation may affect the number of sperm in the epididymis and the weight and morphology of the testicles and epididymis due to histopathological changes in EMR-exposed testicles.
Koca et al.Citation13 investigated the harmful effects of EMR radiating from mobile phones on rat kidneys by both light and electron microscopies. Light microscope findings included damage in the glomerulus, dilatation in Bowman’s capsule, large gaps between tubules that were damaged, perivascular edema and inflammatory cell infiltration. In electron microscopic examination, they observed thickening in the basal membrane, irregularity in the capillary endothelium and severed connections between tubules. In our investigation of EMF-exposed rats’ kidney tissues, mild dilatation in Bowman’s capsule, mild degeneration in tubular structures and increased congestion were observed.
Caspase-3 activity depends on cytosolic Ca+2 concentration. Ca+2 is the main ion involved in various biological processes such as hormone secretion and mitotic division.Citation8 In severe Ca+2 regulation disorders, occurrence of complete necrosis leads to cellular death. However, if the intracellular increase in Ca+2 concentration is more controlled through the apoptosis, stimulations characterized by mild degenerations and the regulation of cellular death are observed.Citation21 In our study, although Ca+2 levels in the study group slightly decreased compared to than those of the control group, no statistically significant difference was observed. In our comparison of total plasma K+ values of blood samples from the control and exposed groups, we observed a statistically significant difference. It is thought that high plasma K+ levels occur due to damage in the kidneys of EMF-exposed rats.
Ozguner et al.Citation22 demonstrated the occurrence of free radicals in relation to mobile phone usage and their negative effects on rat kidneys. In Ragy’s study,Citation23 the effect of 900 MHz EMR produced by mobile phones on the brain, liver and kidney tissues of male albino rats was investigated. EMR emitted from mobile phones led to a significant increase in malondialdehyde levels and a significant decrease in total antioxidant capacity levels in kidney tissues. Urea and creatinine were significantly increased in serum (p < 0.05). Ragy reported that the EMR radiating from mobile phones may lead to disorders that cause oxidative stress and biochemical changes in the kidney tissues of albino rats. Although we identified a significant difference between the control and study groups in terms of K+ levels, no statistically significant difference was observed in terms of Na+, CI−, Ca+2, urea and creatinine levels.
Conclusion
This study indicates that the EMR produced by mobile phones has mild histopathological effects on the kidneys of prenatal rats. Mild biochemical changes have been reported in the blood of newborn infants whose mothers were exposed to EMFs during pregnancy. Thus, EMR may have harmful effects on kidneys and could affect the post-natal development of babies.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hancı H, Odacı E, Kaya H, et al. The effect of prenatal exposure to 900-MHz electromagnetic field on the 21-old-day rat testicle. Reprod Toxicol. 2013;42:203–209
- Tumkaya L, Kalkan Y, Bas O, Yilmaz A. Mobile phone radiation during pubertal development has no effect on testicular histology in rats. Toxicol Ind Health. 2013 Oct 9. [Epub ahead of print] (doi:10.1177/0748233713500820)
- Trosić I, Pavicić I, Milković-Kraus S, Mladinić M, Zeljezić D. Effect of electromagnetic radiofrequency radiation on the rats' brain, liver and kidney cells measured by comet assay. Coll Antropol. 2011;35(4):1259–1264
- Tunik S, Ayaz E, Akpolat V, et al. Effects of pulsed and sinusoidal electromagnetic fields on MMP-2, MMP-9, collagen type IV and E-cadherin expression levels in the rat kidney: An immunohistochemical study. Anal Quant Cytol Histol. 2013;35(5):253–260
- Bartsch H, Bartsch C, Seebald E, et al. Chronic exposure to a GSM-like signal (mobile phone) does not stimulate the development of DMBA-induced mammary tumors in rats: results of three consecutive studies. Radiat Res. 2002;157(2):183–190
- Leszczynski D, Joenvaara S, Reivinen J, Kuokka R. Non-thermal activation of the hsp27/p38MAPK stres pathway by mobile phone radiation in human endothelial cells: Molecular mechanism for cancer- and blood-brain barrier related effects. Differentiation. 2002;70(2–3):120–129
- Flütsch A, Ackermann R, Schroeder T, et al. Combined inhibition of caspase-3 and caspase-7 by two highly selective DARPins slows down cellular demise. Biochem J. 2014;461(2):279–290
- Giorgi C, Romagnoli A, Pinton P, et al. Ca+2 signaling, mitochondria and cell death. Curr Mol Med. 2008;8(2):119–130
- Choi DW. Calcium stil center-stage in hypoxic ischemic neuronal death. Trends Neurosci. 1995;18(2):58–60
- Tombal B, Denmeade SR, Isaacs JT. Assessment and validation of a microinjection method for kinetic analysis of [Ca+2] in individual cells undergoing apoptosis. Cell Calcium. 1999;25(1):19–28
- Koca O, Gokce AM, Akyuz M, Ercan F, Yurdakul N, Karaman MI. A new problem in inflammatory bladder diseases: Use of mobile phones! Int Braz J Urol. 2014;40(4):520–525
- Meral I, Mert H, Mert N, et al. Effects of 900-MHz electromagnetic field emitted from cellular phone on brain oxidative stres and some vitamin levels of guinea pigs. Brain Res. 2007;1169:120–124
- Koca O, Gökçe AM, Öztürk MI, Ercan F, Yurdakul N, Karaman MI. Effects of intensive cell phone (Philips Genic 900) use on the rat kidney tissue. Urol J. 2013;10(2):886–891
- Oktem F, Ozguner F, Mollaoglu H, Koyu A, Uz E. Oxidative damage in the kidney induced by 900-MHz-emitted mobile phone: Protection by melatonin. Arch Med Res. 2005;36(4):350–355
- Ono T, Saito Y, Komura J, et al. Absence of mutagenic effects of 2.45 GHz radiofrequency exposure in spleen, liver, brain, and testis of lac Z-transgenic mouse exposed in utero. Tohoku J Exp Med. 2004;202(2):93–103
- Nakamura H, Matsuzaki I, Hatta K, Nobukuni Y, Kambayashi Y, Ogino K. Non thermal effects of mobile-phone frequency microwaves on uteroplacental functions in pregnant rats. Reprod Toxicol. 2003;17(3):321–326
- Yakymenko I, Sidorik E. Risks of carcinogenesis from electromagnetic radiation of mobile telephony devices. Exp Oncol. 2010;32(2):54–60
- Dasdag S, Ketani MA, Akdag Z, et al. Whole body microwave exposure emitted by cellular phones and testicular function of rats. Urol Res. 1999;27:219–223
- Paurlis AF. Reproductive and developmental effects of EMF in vertebrate animal models. Pathophysiology. 2009;16(2–3):179–189
- Akdag MZ, Celik MS, Ketani A, Nergiz Y, Deniz M, Dasdag S. Effect of chronic low-intensity microwave radiation on sperm count, sperm morphology, and testicular and epididymal tissues of rats. Electromagn Biol Med. 1999;18:133–145
- Pinton P, Giorgi C, Siviero R, et al. Calcium and apoptosis: ER-mitochondria Ca+2 transfer in the control of apoptosis. Oncogene. 2008;27(50):6407–6418
- Ozguner F, Oktem F, Ayata A, Koyu A, Yilmaz HR. A novel antioxidant agent caffeic acid phenethyl ester prevents long term mobile phone exposure-induced renal impairment in rat. Prognostic value of malondialdehyde, N-acetyl-beta-D glucosaminidase and nitricoxide determination. Mol Cell Biochem. 2005;277(1):73–80
- Ragy MM. Effect of exposure and withdrawal of 900-MHz-electromagnetic waves on brain, kidney and liver oxidative stress and some biochemical parameters in male rats. Electromagn Biol Med. 2014 Apr 8. [Epub ahead of print]. (doi:10.3109/15368378.2014.906446)