Abstract
The objective of our study is to research biochemically and histopathologically the effect of nimesulide on oxidative damage inflicted by ischemia–reperfusion (I/R) on the rat renal tissue. Twenty-four albino Wistar type of male rats were used for the experiment. The animals were divided into groups as: renal ischemia–reperfusion control (RIR), nimesulide + renal ischemia–reperfusion of 50 mg/kg (NRIR-50), nimesulide + renal ischemia–reperfusion of 100 mg/kg (NRIR-100), and sham groups (SG). In NRIR-50 and NRIR-100 groups were given nimesulide, and RIR and SG groups were given distilled water, an hour after anesthesia. Groups, except for the SG group, 1-h-ischemia and then 6-h-reperfusion were performed. In the renal tissue of the RIR group in which the malondialdehyde (MDA), myeloperoxidase (MPO), and 8-hydroxyguanine (8-OHGua) levels were measured, the COX-1 and COX-2 activities were recorded. Nimesulide at 100 mg/kg doses reduced the oxidant parameters more significantly than 50 mg/kg doses; on the other hand, it raised the antioxidant parameters. It has been shown that 100 mg/kg doses of nimesulide prevented the renal I/R damage more significantly than a dose of 50 mg/kg, which shows that nimesulide, in clinics, could be used in the prevention of I/R damage.
Introduction
Renal ischemia occurs during various urological vascular surgical procedures.Citation1 Ischemia is the occurrence of oxygen deprivation in the tissue due to a reduction or a complete cessation in the blood stream reaching the tissue.Citation2 The long-term oxygen deprivation in the organs and tissues leads to necrosis (death of cells). For this reason, the first response/intervention to be made on the tissue with ischemia is to enable the tissue to be reperfused. However, the reperfusion of ischemic kidneys gives rise to an increase in the malondialdehyde (MDA) and myeloperoxidase (MPO) levels, which injure the renal tissue to a greater extent, and a decrease in antioxidant levels, such as glutathione (GSH).Citation3 Reactive oxygen species (ROS) released by the polymorphonuclear leukocytes (PMNLs) settling into the tissue during reperfusion increase the tissue damage further.Citation4 These ROS known as reperfusion mediators lead to lipid peroxidation and the oxidative damage of the cell DNA.Citation5–7
Another source of the ROS, on the other hand, is the cyclooxygenase-2 (COX-2) enzyme which becomes active during the period of ischemia–reperfusion (I/R).Citation8 COX-2 enzyme also plays a major role in the production of proinflammatory prostaglandins derived from arachidonic acid and in the induction of inflammation.Citation9,Citation10 Another isoform of COX enzyme is cyclooxygenase-1 (COX-1). COX-1 is a structural enzyme responsible for the cytoprotective activity in the tissues.Citation9 The literary information suggests that the I/R damage is a complicated pathological process starting with the oxygen deprivation of the tissue, pushing ahead with the production of ROS and expanding with the inflammatory response. Therefore, the medications inhibiting both the antioxidant and the COX-2 enzymes selectively are thought to be of use in the prevention of I/R damage. Nimesulide, which we are going to try out in our study, is an anti-inflammatory, analgesic and antipyretic COX-2 selective inhibitor drug.Citation11 It has been experimentally demonstrated that nimesulide protects the gastric and brain tissues against oxidative damage.Citation12,Citation13
This information acquired from the literature suggests that nimesulide is a drug with both antioxidant and selective anti-inflammatory effects. In the literature reviews, no information regarding the protective effect of nimesulide on the renal I/R damage was found. Hence, the objective of our study is to research biochemically and histopathologically the effect of nimesulide on oxidative damage inflicted by I/R on the rat renal tissue.
Materials and methods
Experimental animals
A total of 24 Albino Wistar type of male rats were used in this study, the weights of which varied between 220–235 g. Animals were obtained from the Medical Experimental Practice and Research of Ataturk University. The animals were accommodated and fed at a room temperature of 22 °C in the form of pre-experimental groups.
Chemical substances
Nimesulide utilized during the experiment was provided by DEVA (Turkey); Thiopental Na, on the other hand, was provided by I.E. ULAGAY (Turkey).
The general procedure
Prior to the applications of surgical and pharmacological procedures, the experimental animals were divided into four groups as: renal ischemia–reperfusion control group (RIR), the group with nimesulide + renal ischemia–reperfusion of 50 mg/kg (NRIR-50), the one with nimesulide + renal ischemia–reperfusion of 100 mg/kg (NRIR-100), and also the sham groups (SG) on which sham surgery would be performed.
Surgical and pharmacological procedures
The surgical procedures on the rats were performed under sterile conditions in a suitable laboratory environment by applying a 25 mg/kg of intraperitoneal (i.p.) thiopental sodium anesthesia. An hour before the thiopental sodium anesthesia, 50 mg/kg doses of nimesulide was given to the animals in NRIR-50 group (n = 6), while 100 mg/kg doses of nimesulide was given to those in NRIR-100 group (n = 6) orally through the use of a catheter. For the RIR and SG groups of rats, on the other hand, distilled water was given as a solvent through the same method. In the wake of the thiopental sodium injection, the rats were kept waiting for the manifestation of the appropriate period when the surgical procedure would be performed. The period of time the animals remained inactive in a supine position is regarded as an appropriate time span for the surgical attempt.Citation3 Within this period of time, the unilateral left kidneys of all the rats were reached through the incision on their dorsal sections. Afterwards, except for the SG, 1-h-ischemia was generated by attaching clips to the renal artery and the veins reaching the kidney. At the end of this duration, 6-h-reperfusion (by detaching vein clips) was provided for RIR, NRIR-50, and NRIR-100 groups of rats, after which the kidneys of RIR, NRIR-50, and NRIR-100 groups were excised by killing the rats by means of an overdose of anesthesia. Biochemical and histopathological examinations were performed on the excised kidneys. The biochemical and histopathological results obtained from the NRIR-50 and NRIR-100 groups were evaluated by comparing them with those obtained from the RIR and SG groups.
The biochemical analysis of the renal tissue
In order to assess the enzyme activities in the renal tissues, homogenates from the renal tissues were prepared. The amounts of tGSH and MDA, and the MPO enzyme activities in the supernatants obtained from these homogenates were determined by utilizing proper methods based on the literature.
Preparation of samples
At this stage of the study, 0.2 g of each renal tissue excised was weighed out. The tissues were homogenized in an icy atmosphere by respectively being complemented with 2 mL within the potassium phosphate buffer with pH = 6 containing 0.5% of hexadecyltrimethylammonium bromide (HDTMAB) is used for MPO assay, a 1.15% of potassium chloride solution for MDA assay, and phosphate buffer with pH = 7.5 for the other measurements.
Later on, they were centrifuged at +4 °C at 10,000 rpm for 15 min. The supernatant part was used as a sample for analysis.
Assay for MDA
It is based on the fact that the absorbance of the pink complex generated by thiobarbituric acid (TBA) and MDA at high temperatures (95 °C) is spectrophotometrically measured at a wavelength of 532 nm.Citation14
Assay for MPO activity
In the identification of MPO enzyme activity, the oxidation reaction was performed by means of MPO-mediated H2O2, where the solution of 4-aminoantipyrine/phenol exists as a substrate.Citation15
Assay for total glutathione (tGSH)
5,5′-Dithiobis (2-nitrobenzoic acid) (DTNB) in a measuring environment is a disulphide chromogen and is readily reduced/minimized by compounds of sulfhydryl groups. The resultant yellow color is measured spectrophotometrically at 412 nm.Citation16
DNA oxidation analysis
Preparation of the tissue
The 50–200 mg of the tissue excised from the kidney was homogenized in ice or at +4 °C with a homogenization buffer (30 mM Tris pH 8, 10 mM EDTA, 10 mM 2-mercaptoethanol, 0.5% (v/v) Triton X-I00) by means of a mechanical homogenizer.
Afterwards, by centrifuging this mixture at 1000g for 10 min, the supernatant part of it was removed. The pellet obtained was suspended once again with a 1 mL extraction buffer (0.1 M Tris pH 8, 0.1 M NaCI, 20 mM EDTA) and homogenized through vortexing it for 30 s. Later on, it was centrifuged for 2 min at 1000g. The pellet obtained was re-suspended with an extraction buffer.
The suspension process takes place by vortexing in order to completely blend. Four hundred microliters of phenol are added into the mixture and is vortexed robustly for a minute. Then, it is kept for 10 min at a room temperature in order for the phases to disperse, after which the upper phase is separated and put into a clean tube. Four hundred microliters of chloroform–isopropanol are added into the portion taken into the clean tube (24:1) and is centrifuged at 1000g for 10 min, after which the upper phase is again put into a clean tube. During this process, contamination must be avoided. By adding 40 µL of 3 M sodium acetate (pH 5) and 800 µL of ice-cold ethanol into the mixture obtained from the last centrifuge, the mixture is blended well through the gradual tossing up and down. It is centrifuged for 15 min at 1000g, and the portion remaining at the top is thoroughly removed and 1 mL of 70% ethanol is added to the portion left at the bottom.Citation17
DNA hydrolysis process through formic acid
Finally, 0.5 mL of 60% formic acid is added into the 1 mL of mixture obtained. The lids of the tubes are closed. They are kept at 150 °C for 60 min. The tubes are kept aside for a while to let them get cool. They are preserved at the room temperature to allow the formic acid in the cooling tubes to move away, and almost 1 mL of mixture is preserved at −20 °C until the business day.Citation18
The analysis of 8-hydroxyguanine through HPLC
8-Hydroxyguanine (8-OHdG) and dG levels were measured in HPLC with the help of HPLC-UV and HPLC-ECD electrochemical detectors of various wavelengths through the predefined (default) systems. Prior to the HPLC analysis, the hydrolyzed DNA samples were solved once again with the HPLC eluent. The final volume was proved to be 1 mL. Twenty microliters of final hydrolysate HPLC-ECD were injected (HP, HP 1049A ECD detector, Agilent 1100 modular systems HP 1049A ECD detector, Germany); reversed phase C18 (RP-C18) analytical column was utilized (250 mm × 4.6 mm × 4.0 µm, Phenomenex, CA). The mobile phase consisted of a 0.05 M of potassium phosphate (pH 5.5) buffer containing acetonitrile (97: 3, v/v) with a flow rate of 1 mL/min. By measuring the absorbance of dG concentration at 245 nm, 8-OHdG was observed through the electrochemical reading (600 mV). dH and 8-OHdG were determined by using the dG and 8-OHdG standards of the Sigma brand. 8-OHdG7 dG106 was assigned as the DNA damage marker.Citation19,Citation20
Analysis of COX activity
COX Activity Assay Kit measures the peroxidase activity of COX. The principle of the reaction is based on the colorimetric evaluation of the formation of oxidized N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) at 590 nm.
Preparation of reactives
Assay buffer
Three milliliters of an assay buffer were prepared by being diluted with 27 mL of HPLC-grade water.
Hem reactive
Eighty-eight microliters of hem solution were diluted with a 1.912 mL of assay buffer prepared beforehand.
Arachidonic acid solution
Hundred microliters KOH were added into 100 µL of arachidonic acid and they were blended through vortexing. This mixture was diluted with 1.8 mL of HPLC-grade water. In addition to these reactives, the COX standard, colorimetric substrate, DuP-697 (COX-2 inhibitor), and SC-560 (COX-1 inhibitor) were ready for use within the kit.
Performing the experiment
On the wells of the microplate containing 96 wells were samples marked, for each of which a sample blind/blank, an inhibitor and standard wells were marked. The samples were taken out of the fridge and after dissolving, 50 µL was taken from each sample for the sample blind and was transferred into the microcentrifuge tubes. The tubes were boiled in the boiling water-bath for 5 min, after which they were centrifuged for a minute at 8000g in the microcentrifuge. The supernatant was utilized as a sample blind. Both 150 µL of assay buffer and 10 µL of hem solution were put into the COX standard well, sample and sample blind wells.
Following this, 10 µL from the standard, sample and inactive samples was, respectively, added into the wells. On the other hand, 140 µL of assay buffer, 10 µL of hem solution, 10 µL of sample, and 10 µL of SC-560 solution were added into the inhibitor wells. After they were shaken in the plate shakers for a few seconds, they were left to incubation for 5 min at 25 °C. In the wake of the incubation, first, 20 µL of colorimetric substrate and then 20 µL of arachidonic acid solution were added into all the wells. The plate was mixed for a few seconds in the mixer/liquidizer, and after incubation for 5 min at 25 °C, the absorbances at a wavelength of 590 nm were read. The total COX activity and COX-1 and COX-2 activities were calculated with the help of the formula presented below. Having calculated, the total COX activity for each sample and the COX activities of the samples treated via SC-560 were calculated with the same formula. These results were the COX-2 activities of the samples. The COX-1 activity results of the samples were obtained by extracting the COX-2 activities from the total COX activities. The amount of enzyme oxidizing 1 nmol TMPD in a minute at 25 °C was taken as 1 enzyme unit, and the enzyme activities in the samples were given as enzyme unit per gram, age and tissue.
TV: total volume, SV: sample volume,
*In order to reduce PGG2 to PGH2 2 mol TMPD is consumed. Thus, the results were divided in two.
Histopathological procedures
The kidneys were fixed in the formalin solution of 10% neutral buffer. Incisions of 4 µm thickness were obtained from the paraffin blocks prepared after the routine tissue follow-up/tracking. These incisions were dyed with hematoxylin eosin (H&E) and periodic acid-Schiff (PAS) stains. The histopathological assessment was performed through the light microscope by a pathologist uninformed of what sort of treatment had been applied to the animals (Olympus BX 51, Tokyo, Japan). The most sensitive region in the kidneys, in terms of ischemic damage, was assessed as the renal cortex and renal medulla, notably the outer zone of renal medulla. The histological sections of the kidneys were evaluated in terms of tubular necrosis, intratubular cast formation, inflammation of the renal interstitium, presence of apoptotic bodies, swelling and brush border loss in the kidney tubular epithelium.
Statistical analyses
The results acquired from the experiments were expressed as “average value ± standard error”(x ± SEM). The degree of importance of the difference between groups was determined by utilizing the one-way analysis of variance (ANOVA) test, after which a least significant difference (LSD) test was performed. All the statistical processes were performed in the statistical program, “IBM SPSS Statistics Version 20”, and p < 0.05 value was regarded as significant.
Results
Biochemical findings
RIR, NRIR-50, NRIR-100, and SG groups on MDA levels in rat renal tissue
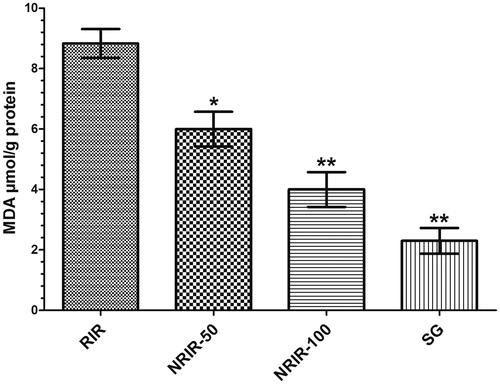
As seen in , MDA levels in RIR group was increased significantly but were decreased in NRIR-50 (8.8 ± 0.47 µmol/g protein, p < 0.05), NRIR-100 (6.0 ± 0.57 µmol/g protein, p < 0.0001) and SG groups ().
RIR, NRIR-50, NRIR-100, and SG groups on MPO levels in rat renal tissue
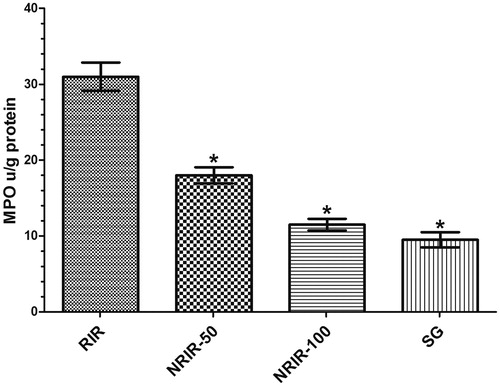
The MPO levels in the RIR group were measured higher than NRIR-50, NRIR-100, and SG groups (, ).
RIR, NRIR-50, NRIR-100, and SG groups on tGSH levels in rat renal tissue
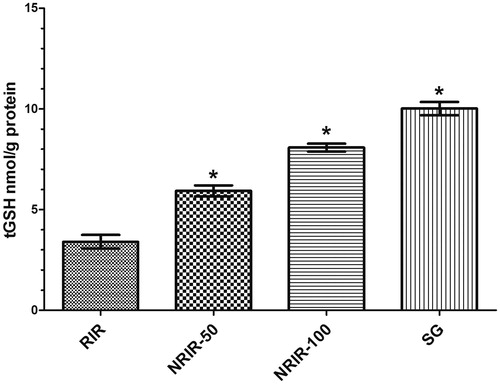
The rate of tGSH was seen highly significant in all groups except RIR (p < 0.0001) (, ).
RIR, NRIR-50, NRIR-100, and SG groups on 8-OHGua levels in rat renal tissue
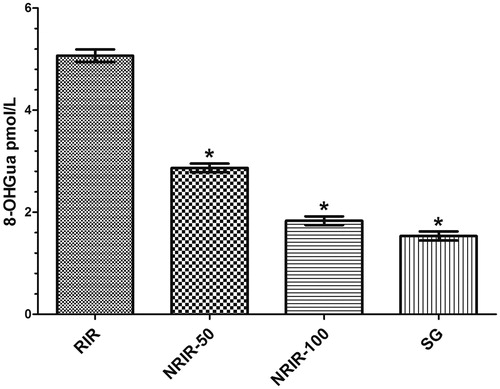
As is seen, the 8-OHGua levels in NRIR-50, NRIR-100, and SG groups were lower than in the RIR group (p < 0.0001) (, ).
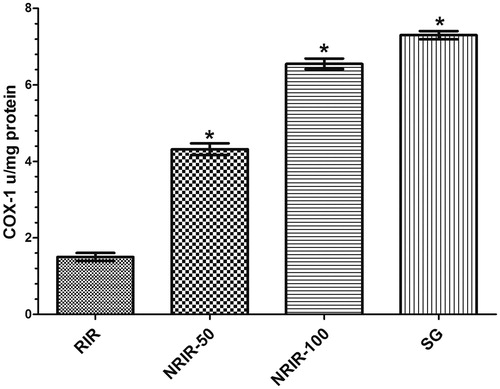
COX-1 activity in RIR, NRIR-50, NRIR-100, and SG groups in rat renal tissue
As seen in , COX-1 activity in NRIR-50, NRIR-100, and SG groups was compared with RIR group and was seen little more significant (p < 0.0001, ).
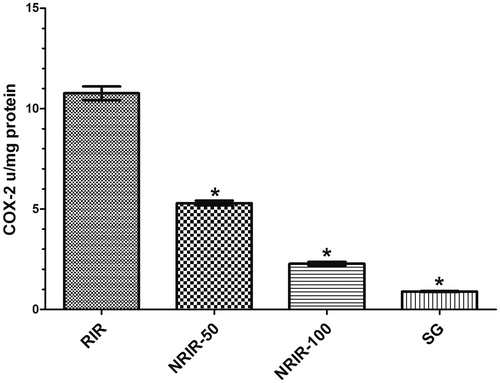
COX-2 activity in RIR, NRIR-50, NRIR-100, and SG groups in rat renal tissue
COX-2 activity was seen more significantly low in NRIR-50, NRIR-100, and SG groups (p < 0.0001) (, ).
Histopathological findings
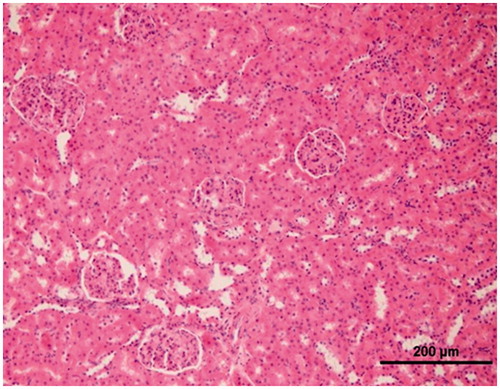
Histopathological evaluation of the renal tissue of sham animal group
As it is seen in , in the histopathological evaluation of the renal tissue of sham animal group, the normal view was monitored (H&E, X20).
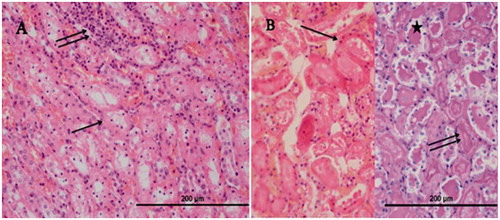
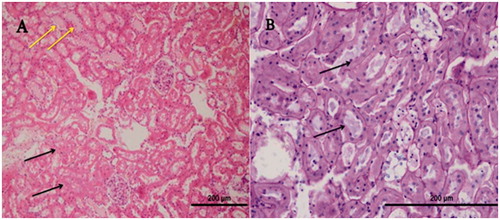
Histopathological evaluation of the renal tissue of RIR group
In the outer zone of renal medulla of the group RIR, a distinctively tubular (in the tubular epithelium) necrosis (, single arrow, H&E, X40), inflammation in the renal interstitium (, double arrow, H&E, X40), and numerous apoptotic bodies (, arrows, PAS-H&E, X40), hyalin cast accumulation in dilated tubuli (, star, PAS-H&E, X40), and also brush border loss in the proximal renal tubular epithelial cells (, PAS-H&E, X40) were ascertained.
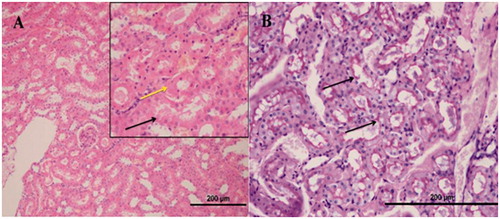
Histopathological evaluation of the renal tissue of NRIR-50 group
In the histopathological sections of the kidneys of NRIR-50 group of rats, tubular necrosis (, black arrow, H&E, X20), hyalin cast accumulation in dilated tubuli (, yellow arrow, H&E, X20), a minority of apoptotic bodies, and swelling in renal tubular epithelial cells (, H&E, X20) were monitored. No inflammation was seen in the renal interstitium (, H&E, X20). A brush border loss in the proximal renal tubular epithelial cells (, arrows, PAS, X40) was monitored.
Histopathological evaluation of the renal tissue of NRIR-100 group
In the renal sections of NRIR-100 group of rats, swelling in the renal tubular epithelial cells (, black arrow, H&E, X40) and a rare tubular necrosis (, yellow arrow, H&E, X40) were monitored, whereas hyalin cast accumulation in tubuli, apoptotic bodies, or inflammation in the renal interstitium (, H&E, X20) was not seen. It was monitored that the brush borders were protected in the proximal tubuli (, PAS, X40).
Discussion
In this study, the effect of nimesulide on oxidative damage inflicted by I/R on the rat renal tissue was biochemically and histopathologically researched. As it is known, a series of chemical events progressing up to the death of cells in the ischemic tissue occurs.Citation21 The fact that the blood abundantly oxygenated through reperfusion turns into an ischemic tissue starts the complex process of reactions accompanied by the neutrophiles and ROS which damages the tissue to a greater extent.Citation2 The most harmful effect ROS initiates within the cell is lipid peroxidation. MDA is the end-product of the lipid peroxidation, and causes the cell damage to exacerbate more.Citation22 In our study, the fact that the amount of MDA in the renal tissue of the RIR group is found significantly higher than the healthy renal tissue suggests that the I/R process leads to an oxidative stress. There are studies demonstrating that the amount of MDA in the renal I/R damage gets significantly high compared to a healthy tissue.Citation3 Also in MPO activity in the renal tissue of the RIR group, a significant increase in comparison to the sham group was determined. MPO secreted from the PMNL leads to the production of ROS, such as , H2O2, and OH√ and transforms H2O2 into hypochlorous acid radical (HOCl), which is a powerful oxidant, in the presence of Cl ion.Citation23,Citation24 Tok et al. also reported that the MPO activity in the renal tissue where I/R was performed significantly rose when compared with a healthy tissue.Citation3 Again, it was ascertained that the amount of GSH in the renal tissue of the RIR group was found to be lower than the sham group. GSH is an endogenous antioxidant molecule which protects the cell membrane lipids, proteins, nucleic acid, and carbohydrates against the oxidant damage.Citation25,Citation26 The fact that the amount of GSH diminishes in I/R damage was reported in the previous studies.Citation3
The excessive production of ROSs may readily damage the DNA molecule.Citation27 The OH√ radical easily reacts with deoxyribose and bases. It gives rise to the DNA damage by extracting hydrogen from nucleic acids or reacting with double bonds.Citation28
8-OHGua was regarded as an important marker representing the DNA oxidation.Citation29 Our test results suggested that the amount of 8-OHGua in the renal tissue of the RIR group on which we had generated I/R significantly increased higher than the sham group, which explains that the DNA oxidation in the renal tissue of the RIR group is more severe than the sham group. It was stated that the amount of 8-OHGua in I/R and other tissue damage models rose in parallel to the increase of the oxidant parameters.Citation30,Citation31
Table 1. MDA level, MPO activity, tGSH, and 8-OHGua levels in the RIR, NRIR-50, NRIR-100, and SG groups.
Table 2. COX-1 and COX-2 activity in RIR, NRIR-50, NRIR-100, and SG groups.
In the renal tissue of the RIR group, in which the MDA, MPO, and 8-OHGua levels proved to be high, a decrease in the COX-1 activity and a rise in the COX-2 activity were recorded. COX-1 is responsible for the synthesis of cytoprotective prostaglandins.Citation32 The inhibition of this enzyme brings about a decline in the production of cytoprotective prostaglandins and, thus, a tissue damage.Citation33 It was reported that the COX-2 activity in the inflamed region showed a prominent increase.Citation34
Isaoglu et al. also pointed out in their studies that there was a decrease in COX-1 activity and an increase in COX-2 activity in the ovary tissues where I/R was generated; separately, in this study, the oxidant parameters were found to be high, whereas the antioxidant ones proved to be low in the damaged tissue where COX-1 activity was low and COX-2 activity was found high.Citation35 A noticeable degree of necrosis was determined in the proximal tubular epithelium of the outer zone of renal medulla of the RIR group where oxidant parameters were found to be much higher. The studies show that the tubular necrosis is a pathological event that occurs as the result of the ischemic damage.Citation36 Furthermore, a histopathologically severe inflammation was found in the renal tissue of the RIR group. Inflammation plays a major role in the exacerbation of the tubular necrosis; the inflammation is stated to result from the inflammatory mediators secreted by the damaged tubular cells.Citation37 Again, it was observed that in the renal tissue of the RIR group, the number of apoptotic cells significantly increased when compared with the sham group. It was demonstrated that hypoxia, I/R damage, and oxidative stress led to apoptosis.Citation38 In another study, proinflammatory cytokines were reported to cause apoptosis to increase by activating the caspase enzymes.Citation39 Şener et al. put forward that the oxidative stress enhanced apoptosis in the renal tissue, whereas the antioxidant treatment declined.Citation40 A hyalin cast accumulation in dilated tubuli and a brush border loss in the proximal renal tubular epithelial cells in the renal tissue of the RIR group were monitored. It is also expressed in the literatures that the oxidative stress lead to a noticeable hyalin cast accumulation in dilated tubuli and a brush border loss in the epithelial cells.Citation3 In ischemia, in the proximal tubular cells of the renal tissue, the destruction of the proteins like spectrin and ankyrin in the cytoplasm increase; this event causes the brush borders of the tubular cells to break off.Citation41,Citation42
As also seen from our test results, nimesulide at 100 mg/kg doses reduced the oxidant parameters more significantly than 50 mg/kg doses; on the other hand, it raised the antioxidant parameters. Nimesulide was reported to protect the brain tissue from the oxidative damage induced by ischemia.Citation12 Moreover, nimesulide was said to reduce the MDA, MPO, and COX-2 enzyme levels and to enhance the GSH and COX-1 levels in the ischemic tissue.Citation35 It was demonstrated that nimesulide inhibited the occurrence of inflammation and edema by suppressing the PMNL adhesion responsible for the I/R damage through integrin CD11b/CD18 and L-selectin.Citation11 No pathological finding (inflammation in the renal interstitium, necrosis, numerous apoptotic bodies, hyalin cast accumulation in dilated tubuli and brush border loss in the proximal tubular epithelial cells), apart from a slight swelling and hemorrhage, was encountered in the tubular cells of the rat renal tissue to which 100 mg/kg doses of nimesulide were given. It was also shown in the literatures that the oxidative stress brought about hyalin cast accumulation in dilated tubuli and brush border loss in the proximal tubular epithelial cells in the renal tissue.Citation3
Conclusions
Nimesulide at 100 mg/kg doses prevented the I/R renal damage more significantly than 50 mg/kg doses. The fact that nimesulide has the characteristic of an antioxidant and a COX-2 selective inhibitor suggests that there will be fewer side-effects to it and that it could be applied in clinics in the prevention of I/R damage.
Supplementary material available online
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Conesa EL, Valero F, Nadal JC, et al. N-acetyl-L-cysteine improves renal medullary hypoperfusion in acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2001;281:730–737
- Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am. 1992;72:65–83
- Tok A, Sener E, Albayrak A, et al. Effect of mirtazapine on oxidative stress created in rat kidneys by ischemia-reperfusion. Ren Fail. 2012;34:103–110
- Eltzschig HK, Collard CD. Vascular ischemia and reperfusion injury. Br Med Bull. 2004;70:71–86
- Del Maestro RF. An approach to free radicals in medicine and biology. Acta Physiol Scand Suppl. 1980;492:153–168
- Huang HY, Helzlsouer KJ, Appel LJ. The effects of vitamin C and vitamin E on oxidative DNA damage: Results from a randomized controlled trial. Cancer Epidemiol Biomarker Prev. 2000;9:647–652
- Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–370
- Miettinen S, Fusco FR, Yrjanheikki J, et al. Spreading depression and focal brain ischemia induce cyclooxygenase-2 in cortical neurons through N-methyl-D-aspartic acid-receptors and phospholipase A2. Proc Natl Acad Sci U S A. 1997;94:6500–6505
- Suleyman H, Demircan B, Karagoz Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol Rep. 2007;59:247–258
- Vilanova JM, Figueras-Aloy J, Rosello J, Gomez G, Gelpi E, Jimenez R. Arachidonic acid metabolites in CSF in hypoxic-ischemic encephalopathy of newborn infants. Acta Paediatr. 1998;87:588–592
- Suleyman H, Cadirci E, Albayrak A, Halici Z. Nimesulide is a selective COX-2 inhibitory, atypical non-steroidal anti-inflammatory drug. Curr Med Chem. 2008;15:278–283
- Al-Majed AA, Al-Yahya AA, Asiri Y, Al-Gonaiah MA, Mostafa AM. Nimesulide prevents oxidative stress damage following transient forebrain ischemia in the rat hippocampus. Res Commun Mol Pathol Pharmacol. 2004;115–116:49–62
- Altinkaynak K, Suleyman H, Akcay F. Effect of nimesulide, rofecoxib and celecoxib on gastric tissue glutathione level in rats with indomethacin-induced gastric ulcerations. Pol J Pharmacol. 2003;55:645–648
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358
- Wei H, Frenkel K. In vivo formation of oxidized DNA bases in tumor promoter-treated mouse skin. Cancer Res. 1991;51:4443–4449
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205
- Shigenaga MK, Aboujaoude EN, Chen Q, Ames BN. Assays of oxidative DNA damage biomarkers 8-oxo-2′-deoxyguanosine and 8-oxoguanine in nuclear DNA and biological fluids by high-performance liquid chromatography with electrochemical detection. Methods Enzymol 1994;234:16–33
- Kaur H, Halliwell B. Measurement of oxidized and methylated DNA bases by HPLC with electrochemical detection. Biochem J. 1996;318:21–23
- Asami S, Hirano T, Yamaguchi R, Tomioka Y, Itoh H, Kasai H. Increase of a type of oxidative DNA damage, 8-hydroxyguanine, and its repair activity in human leukocytes by cigarette smoking. Cancer Res. 1996;56:2546–2549
- Floyd RA, Watson JJ, Wong PK, Altmiller DH, Rickard RC. Hydroxyl free radical adduct of deoxyguanosine: Sensitive detection and mechanisms of formation. Free Radic Res Commun. 1986;1:163–172
- Semenza GL. Cellular and molecular dissection of reperfusion injury: ROS within and without. Circ Res. 2000;86:117–118
- Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39:1529–1542
- Musemeche CA, Henning SJ, Baker JL, Pizzini RP. Inflammatory enzyme composition of the neonatal rat intestine: Implications for susceptibility to ischemia. J Pediatr Surg. 1993;28:788–791
- Otamiri T. Oxygen radicals, lipid peroxidation, and neutrophil infiltration after small-intestinal ischemia and reperfusion. Surgery. 1989;105:593–597
- Rangan U, Bulkley GB. Prospects for treatment of free radical-mediated tissue injury. Br Med Bull. 1993;49:700–718
- Reiter RJ, Melchiorri D, Sewerynek E, et al. A review of the evidence supporting melatonin’s role as an antioxidant. J Pineal Res. 1995;18:1–11
- Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–1828
- Milligan JR, Ward JF. Yield of single-strand breaks due to attack on DNA by scavenger-derived radicals. Radiat Res. 1994;137:295–299
- Grollman AP, Moriya M. Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet. 1993;9:246–249
- Ingec M, Isaoglu U, Yilmaz M, et al. Prevention of ischemia-reperfusion injury in rat ovarian tissue with the on-off method. J Physiol Pharmacol. 2011;62:575–582
- Polat B, Suleyman H, Alp HH. Adaptation of rat gastric tissue against indomethacin toxicity. Chem Biol Interact. 2010;186:82–89
- Madrigal IL, Lopez HS, Alcala FC. Inhıbıdores selectıvos de la cıclooxıgenasa-2: Usos potencıales en perros. Vet Mex. 2002;33:285–307
- Bjarnason I, Zanelli G, Smith T, et al. Nonsteroidal antiinflammatory drug-induced intestinal inflammation in humans. Gastroenterology. 1987;93:480–489
- Krause MM, Brand MD, Krauss S, et al. Nonsteroidal antiinflammatory drugs and a selective cyclooxygenase 2 inhibitor uncouple mitochondria in intact cells. Arthritis Rheum. 2003;48:1438–1444
- Isaoglu U, Yilmaz M, Sener E, et al. The impaired balances of oxidant/antioxidant and COX-1/COX-2 in ovarian ischemia-reperfusion injury and prevention by nimesulide. Lat Am J Pharm. 2012;31:1481–1488
- Lameire N. The pathophysiology of acute renal failure. Crit Care Clin. 2005;21:197–210
- Bonventre JV, Zuk A. Ischemic acute renal failure: An inflammatory disease? Kidney Int. 2004;66:480–485
- Yamamoto K, Tomita N, Yoshimura S, et al. Hypoxia-induced renal epithelial cell death through caspase-dependent pathway: Role of Bcl-2, Bcl-xL and Bax in tubular injury. Int J Mol Med. 2004;14:633–640
- Creagh EM, Conroy H, Martin SJ. Caspase-activation pathways in apoptosis and immunity. Immunol Rev. 2003;193:10–21
- Sener MT, Sener E, Tok A, et al. Biochemical and histologic study of lethal cisplatin nephrotoxicity prevention by mirtazapine. Pharmacol Rep. 2012;64:594–602
- Alejandro VS, Nelson WJ, Huie P, et al. Postischemic injury, delayed function and Na+/K(+)-ATPase distribution in the transplanted kidney. Kidney Int. 1995;48:1308–1315
- Edelstein CL, Wieder ED, Yaqoob MM, et al. The role of cysteine proteases in hypoxia-induced rat renal proximal tubular injury. Proc Natl Acad Sci USA. 1995;92:7662–7666