Abstract
The polyphenol curcumin has several pharmacological effects, including antioxidant, anti-inflammatory and anti-cancer features. In this study, we evaluated the effects of curcumin in cisplatin-induced nephrotoxicity in rats. Male Wistar rats were divided into four groups: (1) control; (2) cisplatin (7 mg/kg body weight, intraperitoneal as a single dose); (3) curcumin (100 mg/kg via gavage, for 10 days); and (4) cisplatin and curcumin. The cisplatin-treated rats exhibited kidney injury manifested by increased serum urea and creatinine (p < 0.05). The kidney tissue from the cisplatin treated rats also exhibited a significant increase in the malondialdehyde (MDA) levels (p < 0.05). The treatment with curcumin prevented a rise in the serum urea, creatinine and MDA levels when compared to the control group kidneys (p < 0.05). The analysis the nicotinamide phosphoribosyltransferase (NAMPT) and sirtuin (SIRT) proteins (SIRT1, SIRT3 and SIRT4), which play important roles in the resistance to stress and the modulation of the threshold of cell death, showed similar trends (p < 0.05). In the cisplatin-only treated rats, the induced renal injury decreased the levels of the NAMPT and SIRT proteins. Conversely, the curcumin increased the levels of the NAMPT and SIRT proteins in the cisplatin-treated rats (p < 0.05). These data suggest that curcumin can potentially be used to reduce chemotherapy-induced nephrotoxicity, thereby enhancing the therapeutic window of cisplatin.
Introduction
Cisplatinum is a powerful antineoplastic drug that is being used in the treatment of many solid tumors, such as those of the head and neck, lung, testis, ovarian and breast cancers.Citation1 Nephrotoxicity is a complication of cisplatin that necessitates dose reduction; however, the mechanism of cisplatin nephrotoxicity is complex. Cisplatin causes oxidative stress, apoptosis and inflammation and increases fibrinogen formation in renal tissue;Citation2–5 and in patients with acute renal failure, the symptomatic lesion is acute necrosis in the proximal tubule. The severity of necrosis is dose, concentration and time dependent.Citation6,Citation7 There are various oxidative stress markers used to identify the damage of the reactive oxygen compounds of the cells, and the malondialdehyde (MDA), which is used to determine lipid peroxidation, is one of them.Citation8 However, there are numerous studies showing that antioxidants prevent or reduce the increase in the MDA levels in tissues that are exposed to oxidative stress.Citation3,Citation5
Curcumin is derived from turmeric (Curcuma longa) plants; its chemical structure was first characterized in 1910, and it is a low-molecular-weight polyphenol of which turmeric plants contain a 2–8% proportion.Citation9 It is a powerful antioxidant that clears –O2, –OH and NO2 radicals and inhibits lipid peroxidation.Citation10,Citation11 Curcumin has been shown to protect kidney cells against oxidative stress via its inhibitory effects on lipid peroxidation, lipid degradation and cytolysis.Citation12
Sirtuins (SIRTs) are a large family of proteins, which may be present in different species, ranging from bacteria to mammals, in various proportions. Seven different SIRT enzymes, encoded by seven different genes, have been identified in mammals. SIRTs are regulatory genes, which control other genes.Citation13 The SIRTs of mammals play important roles in resistance to stress and the modulation of the threshold of cell death, and SIRT1 has been observed to repair DNA breaks due to oxidative stress.Citation14
In mammals, nicotinamide adenine dinucleotide (NAD+) synthesis takes place via a two-stage pathway, which is performed by nicotinamide phosphoribosyltransferase (NAMPT) and nicotinamide/nicotinic acid mononucleotide adenylyltransferase (NMNAT) enzymes. NAD+ is a classical coenzyme that participates in redox reactions and has important biological effects in the cell. SIRT1 plays a regulatory role in NAD+-dependent deacetylation,Citation15 while NAMPT-mediated NAD+ biosynthesis takes part in many processes, including cellular aging, old age, obesity, diabetes and cancer.Citation16,Citation17
This study was designed to evaluate the protective role of curcumin in cisplatin-induced nephrotoxicity and renal SIRT expression.
Materials and methods
Animal materials
This study was performed with the approval of the Firat University Animal Ethics Committee, in accordance with the standard ethical guidelines for experimental animal studies in the Firat University Experimental Research Center (FUDAM). Male Wistar rats, ranging from 195 to 264 grams in weight, which were obtained from FUDAM were used (n = 28, 10 weeks of age). These rats were kept in a laboratory environment of 22 ± 1 °C temperature, and a 12-h light/dark cycle in accordance with ethical guidelines. Their diet consisted of standard pellet feed and tap water.
Experimental design
Cisplatin-induced nephrotoxicity was performed with a single intraperitoneal (i.p.) cisplatin injection. Cisplatin (Sigma Chemical Co., St. Louis, MO) in 0.9% saline (1 ml/100 g/kg i.p.) was injected on the third day of the study in a 7 mg/kg dose, intraperitoneally. The rats were grouped as follows:
Control group (n = 7): cisplatin was not applied, fed a basal diet, third day i.p. isotonic saline injection with an equal volume of cisplatin (1 ml/kg/day).
Cisplatin group (n = 7): cisplatin (Sigma Chemical Co.) in 0.9% saline (1 ml/100 g/kg i.p.) was injected on the third day of the study in a 7 mg/kg dose, intraperitoneally.
Curcumin group (n = 7): cisplatin was not applied, fed a basal diet, third day i.p. isotonic saline injection with an equal volume of cisplatin (1 ml/kg/day). Curcumin (Sigma-Aldrich, St. Louis, MO) was administered two days before and eight days after the injection with a 100 mg/kg dose via gavage, 10 days in total.
Curcumin + cisplatin group (n = 7): cisplatin administered, with curcumin administered two days before and eight days after the cisplatin injection, 100 mg/kg.
Eight days after the cisplatin administration, the rats were decapitated under anesthesia. Tissue samples were stored at −80 °C. The kidneys were removed and perfused with a phosphate buffered solution (PBS, 0.15 M NaCl and 0.01 M sodium phosphate buffer, pH 7.4) through the aorta for histological examination. Blood samples were taken for serum urea nitrogen and creatinine measurements.
Laboratory analyses
Lipid peroxidation was measured in terms of MDA formation, which is the major product of membrane lipid peroxidation done by the Karatepe method with slight modification.Citation18 The MDA concentration of the kidney tissue was measured by high-performance liquid chromatography (Shimadzu, Tokyo, Japan) using a Shimadzu UV–Vis SPD-10 AVP detector and C18- ODS-3, 5 µm, 4.6 × 250 mm column. The mobile phase was 30 mM KH2PO4–methanol (82.5 + 17.5, v/v%, pH 3.6), and the flow rate was 1.2 mL min–1. Chromatograms were monitored at 250 nm, and the injection volume was 20 µL. The homogenized kidney tissue (in PBS) was purified in the presence of 0.01% butylated hydroxytoluene, processed for the analysis of 8-isoprostane as previously described by Wong et al., and determined using ELISA kits (Cayman Chemical, Ann Arbor, MI) according to the manufacturers’ protocols.Citation19
Western blot
Protein extraction was performed by homogenizing the rat kidney in 1 ml ice-cold hypotonic buffer A, containing 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (pH 7.8), 10 mM KCl, 2 mM MgCl2, 1 mM DTT, 0.1 mM EDTA and 0.1 mM phenylmethylsulfonyl-fluoride (PMSF). To the homogenates, 80 µl of 10% Nonidet P-40 (NP-40) solution was added, and the mixture was centrifuged for 2 min at 14,000 g. The supernatant was collected as a cytosolic fraction for HO–1. The precipitates, containing nuclei, were washed once with 500 µl of buffer A plus 40 µl of 10% NP-40, centrifuged, resuspended in 200 µl of buffer C [50 mM HEPES (pH 7.8), 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 0.1 mM PMSF and 20% glycerol] and centrifuged for 5 min at 14,800 g. The supernatant containing nuclear proteins was collected for anti-SIRT1, anti-SIRT3, anti-SIRT4 and anti-pre-B cell colony-enhancing factor (PBEF). Equal amounts of protein (50 µg) were electrophoresed and subsequently transferred to the nitrocellulose membrane (Schleicher and Schuell Inc., Keene, NH). The antibodies against SIRT1, SIRT3, SIRT4 and PBEF were the purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and the primary antibody was diluted (1:1000) in the same buffer containing 0.05% Tween-20. Protein loading was controlled using a monoclonal mouse antibody against b-actin antibody (A5316, Sigma), and the bands were analyzed densitometrically using an image analysis system (Image J; National Institutes of Health, Bethesda, MD).
Statistical analysis
The statistical analysis was performed by using the SPSS, 18.0 software package program (SPSS Inc., Chicago, IL). In order to reveal the differences between the groups, the one-way analysis of variance with a post hoc Tukey’s test was performed. p Value < 0.05 was accepted as a statistical significance.
Results
The serum urea and creatinine concentrations were found to be significantly increased in the cisplatin-treated rats (cisplatin, 397.14 ± 103.78 mg/dL and 4.10 ± 1.63 mg/dL vs. normal control, 45.014 ± 4.77 mg/dL and 0.23 ± 0.08 mg/dL, respectively; p < 0.001; ). However, a marked decrease in the serum urea and creatinine were observed upon administration of the curcumin in the cisplatin-treated rats. lists the alterations in the indicators of oxidative stress, namely, the MDA levels, in the kidney tissues of both the normal and cisplatin-treated rats. To investigate the effects of the cisplatin-induced oxidative stress, we determined the MDA levels, which are well-known biomarkers of the overall oxidative damage to cellular constituents such as membrane lipids. The treatment of the normal animals with curcumin did not affect the lipid peroxidation levels in the kidney tissues (p > 0.05). In the rats treated with cisplatin, the MDA levels of the lipid peroxidation were significantly increased (cisplatin, 2.24 ± 0.40 µmol/L vs. control, 0.35 ± 0.08 µmol/L; p < 0.05). The administration of curcumin with cisplatin diminished the cisplatin-induced increase in the MDA levels in the kidney tissues (1.40 ± 0.30 µmol/L, p < 0.05).
Table 1. Effects of curcumin on serum urea and creatinine levels in rats with cisplatin-induced nephrotoxicity.
Table 2. Effects of curcumin on tissue MDA levels in rats with cisplatin-induced nephrotoxicity.
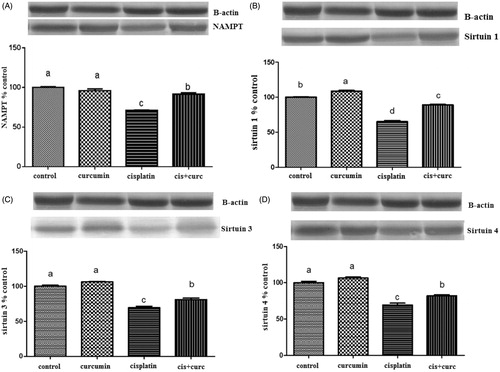
A Western blots analysis of the NAMPT and SIRTs was conducted to determine the effects of the curcumin on the SIRT levels. Representative blots are shown in . Densitometric analysis indicated that the administration of cisplatin markedly decreased the expression of NAMPT, SIRT1, SIRT3 and SIRT4 in the kidney (; p < 0.001). However, the curcumin reversed this effect, and the expressions of NAMPT (panel A), SIRT1 (panel B), SIRT3 (panel C) and SIRT4 (panel D) were increased in the kidneys from the animals treated with cisplatin and curcumin (p < 0.05). No significant change was observed in the normal rats upon treatment with curcumin (; p > 0.05).
Figure 1. The intensity of the bands was quantified by the densitometric analysis. Western blot using the NAMPT (A), SIRT1 (B), SIRT3 (C) and SIRT4 (D) revealed specific bands. B-actin levels were monitored to ensure equal protein loading (bottom panel). Western blot analysis of NAMPT, SIRT1, SIRT3 and SIRT4 in kidney cells in control and curcumin treated rats. Data are percent of the control. The bar represents the standard error of the mean. Blots were repeated at least three times (n = 3) and a representative blot is shown (a–d). Means in the same line without a common superscript differ significantly (p < 0.05). Note: a–dDifferent letters reflects difference with statistical significance (p < 0.05).
Discussion
There are many efforts to reduce the side effects of the potent antineoplastic agent cisplatin that limit its use, especially in preventing nephrotoxicity.Citation3,Citation20–22 Cisplatin is an effective anticancer drug used against lung and ovarian cancer and some lymphomas; however, renal damage has limited its use. Unfortunately, this damage is not specific, and as a DNA intercalating agent, shows considerable dose limiting toxicities.Citation23 Patients undergoing cisplatin therapy frequently suffer from severe toxicities such as nausea, vomiting, renal insufficiency, electrolyte abnormalities, myelosuppression, ototoxicity, peripheral neuropathy, hepatotoxicity and retinopathy, even when administered at standard doses.Citation24 It has been well established that oxidative stress is one of the mechanisms involved in the cell damage induced by cisplatin. Indeed, a decrease in antioxidant defense is clearly observed in in vivo and in vitro experimental models.Citation25 MDA is one of the increased productions of lipid peroxidation biomarkers that are closely associated with the development of cisplatin-induced nephrotoxicity.Citation20
Curcumin is a powerful antioxidant that cleans –O2, OH and NO2 radicals and has been shown to inhibit lipid peroxidation in various animal studies.Citation10,Citation11 It protects kidney cells against oxidative stress by inhibiting lipid peroxidation, lipid degradation and cytolysis.Citation26 Our data showed a significant increase in the MDA levels with cisplatin, and curcumin administration caused a reduction in the MDA levels consistent with previous studies.Citation27,Citation28
In mammals, NAD+ synthesis takes place via a two-stage pathway, which is performed by NAMPT and nicotinamide/nicotinic acid NMNAT enzymes, and the rate-limiting enzyme in this synthesis is NAMPT.Citation29 NAMPT is assumed to be a cytokine-like protein and described as a pre-B colony enhancing factor due to its role in subclinical inflammation.Citation30 In vitro and in vivo studies have shown that decreased ATP synthesis is a determinant of cell death via apoptosis and necrosis.Citation31 Adenosine monophosphate protein kinase is one of the primary regulators maintaining the energy balance, and it detects ATP reduction in the cell.Citation32 In our study, significantly reduced NAMPT levels with cisplatin therapy were found to be increased with curcumin administration (). These findings show that the cellular energy metabolism impaired by cisplatin may be restored with curcumin.
SIRT3 is a soluble protein in the mitochondrial matrix, while the thioredoxin system is a scavenging antioxidant system against mitochondrial hydrogen peroxide (H2O2). SIRT3 has been shown to interact and regulate thioredoxin reductase 2, which is a member of this system. One of the key members of the antioxidant system is cytochrome C, and SIRT3 is essential for cytochrome C expression, which is induced by the peroxisome proliferator-activated receptor gamma-coactivator-1α.Citation33 SIRT4 is located in the mitochondria, and the first 28 amino acids separate after entry into the mitochondria.Citation34 Studies in humans and mice have connected SIRT4 with insulin secretion, which is regulated by food intake.Citation35,Citation36 The most important effect of the SIRT family is the regulation of gene expression. SIRT1 reduces the transcriptional efficiency of P53 by deacetylating the lysine amino acid remnants, and suppressing programmed cell death against oxidative stress and DNA damage.Citation37 At the same time, it regulates the DNA repair capacities of cells and plays a key role in inflammation by regulating NF-kB.Citation38,Citation39 SIRT3 increases cellular respiration and the production of reactive oxygen species. In stress situations, SIRT3 provides the use of acetate as an energy source by activating acetyl coenzyme A synthase 2.Citation40,Citation41 SIRT3 was found to regulate ATP synthesis and increase the gene activity that increases the activity and amount of the antioxidant enzymes.Citation42,Citation43 Although there is restricted knowledge about SIRT4, it is thought to have a protective effect on pancreatic beta cells in calorie restricted mice. One study showed that overexpression of SIRT1 decreases cisplatin-induced cytotoxicity.Citation44 In our study, cisplatin administration caused significant decreases in SIRT1, SIRT3 and SIRT4 levels when compared to the control group (p < 0.05), while curcumin was observed to partially reverse these effects. This result suggests that SIRTs can be possible target to attenuate cisplatin-induced renal damage.
In conclusion, in cisplatin-induced nephrotoxicity, increased MDA and decreased NAMPT, SIRT1, SIRT3 and SIRT4 levels were partially improved by the application of curcumin. These results suggest that curcumin may play a protective role in cisplatin-induced nephrotoxicity and its renoprotective effect is associated to preservation of renal function and redox balance of mitochondria.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Rosenberg B. Fundamental studies with cisplatin. Cancer. 1985;55(10):2303–23l6
- Giaccone G. Clinical perspectives on platinum resistance. Drugs. 2000;59(4)(suppl):S9–S17
- Yilmaz HR, Iraz M, Sogut S, et al. The effects of erdosteine on the activities of some metabolic enzymes during cisplatin-induced nephrotoxicity in rats. Pharmacol Res. 2004;50(3):287–290
- Kaushal GP, Kaushal V, Hong X, Shah SV. Role and regulation of activation of caspases in cisplatin-induced injury to renal tubular epithelial cells. Kidney Int. 2001;60(5):1726–1736
- Lee HS, Kim BK, Nam Y, et al. Protective role of phosphatidylcholine against cisplatin-induced renal toxicity and oxidative stress in rats. Food Chem Toxicol. 2013;58:388–393
- Meyer KB, Madias NE. Cisplatin nephrotoxicity. Miner Electrolyte Metab. 1994;20(4):201–213
- Tanaka H, Ishikawa E, Teshima S, Shimizu E. Histopathological study of human cisplatin nephropathy. Toxicol Pathol. 1986;14(2):247–257
- Zhou H, Kato A, Miyaji T, et al. Urinary marker for oxidative stress in kidneys in cisplatin-induced acute renal failure in rats. Nephrol Dial Transplant. 2006;21(3):616–623
- Esatbeyoglu T, Huebbe P, Ernst IM, Chin D, Wagner AE, Rimbach G. Curcumin—from molecule to biological function. Angew Chem Int Ed Engl. 2012;51(22):5308–5332
- Reddy AC, Lokesh BR. Studies on the inhibitory effects of curcumin and eugenol on the formation of reactive oxygen species and the oxidation of ferrous iron. Mol Cell Biochem. 1994;137:1–8
- Reddy AC, Lokesh BR. Studies on spice principles as antioxidants in the inhibition of lipid peroxidation of rat liver microsomes. Mol Cell Biochem. 1992;111(1–2):117–124
- Cohly HH, Taylor A, Angel MF, Salahudeen AK. Effect of turmeric, turmerin and curcumin on H2O2-induced renal epithelial (LLC-PK1) cell injury. Free Radic Biol Med. 1998;24(1):49–54
- Kelly G. A review of the sirtuin system, its clinical implications, and the potential role of dietary activators like resveratrol: Part 1. Altern Med Rev. 2010;15(3):245–263
- Oberdoerffer P, Michan S, McVay M, et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 2008;135(5):907–918
- Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279(49):50754–50763
- Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898–918
- Garten A, Petzold S, Körner A, Imai S, Kiess W. Nampt: Linking NAD biology, metabolism and cancer. Trends Endocrinol Metab. 2009;20(3):130–138
- Karatepe M. Simultaneous determination of ascorbic acid and free malondialdehyde in human serum by HPLC/UV. LCGC N Am. 2004;22:362–365
- Wong YT, Ruan R, Tay FE. Relationship between levels of oxidative DNA damage, lipid peroxidation and mitochondrial membrane potential in young and old F344 rats. Free Radic Res. 2006;40(4):393–402
- Dogukan A, Tuzcu M, Agca CA, et al. A tomato lycopene complex protects the kidney from cisplatin-induced injury via affecting oxidative stress as well as Bax, Bcl-2, and HSPs expression. Nutr Cancer. 2011;63(3):427–434
- Ulu R, Dogukan A, Tuzcu M, et al. Regulation of renal organic anion and cation transporters by thymoquinone in cisplatin induced kidney injury. Food Chem Toxicol. 2012;50(5):1675–1679
- Pabla N, Dong Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008;73(9):994–1007
- Surendiran A, Balamurugan N, Gunaseelan K, Akhtar S, Reddy KS, Adithan C. Adverse drug reaction profile of cisplatin-based chemotherapy regimen in a tertiary care hospital in India: An evaluative study. Indian J Pharmacol. 2010;42(1):40–43
- Hanigan MH, Devarajan P. Cisplatin nephrotoxicity: Molecular mechanisms. Cancer Ther. 2003;1:47–61
- Chirino YI, Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol. 2009;61(3):223–242
- Cohly HH, Taylor A, Angel MF, Salahudeen AK. Effect of turmeric, turmerin and curcumin on H2O2-induced renal epithelial (LLC-PK1) cell injury. Free Radic Biol Med. 1998;24(1):49–54
- Antunes LM, Darin JD, Bianchi Nde L. Effects of the antioxidants curcumin or selenium on cisplatin-induced nephrotoxicity and lipid peroxidation in rats. Pharmacol Res. 2001;43(2):145–150
- Sahin K, Orhan C, Tuzcu M, et al. Comparative in vivo evaluations of curcumin and its analog difluorinated curcumin against cisplatin-induced nephrotoxicity. Biol Trace Elem Res. 2014;157(2):156–163
- Revollo JR, Grimm AA, Imai S. The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Curr Opin Gastroenterol. 2007;23(2):164–170
- Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor (PBEF)/visfatin: A novel mediator of innate immunity. J Leukoc Biol. 2008;83(4):804–816
- Tsujimoto Y. Apoptosis and necrosis: Intracellular ATP level as a determinant for cell death modes. Cell Death Differ. 1997;4(6):429–434
- Carling D. The AMP-activated protein kinase cascade—a unifying system for energy control. Trends Biochem Sci. 2004;29(1):18–24
- Zhao S, Xu W, Jiang W, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327(5968):1000–1004
- Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460(7255):587–591
- Ahuja N, Schwer B, Carobbio S, et al. Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J Biol Chem. 2007;282(46):33583–33592
- Haigis MC, Mostoslavsky R, Haigis KM, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126(5):941–954
- Vaziri H, Dessain SK, Ng Eaton E, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107(2):149–159
- Jeong J, Juhn K, Lee H, et al. SIRT1 promotes DNA repair activity and deacetylation of Ku70. Exp Mol Med. 2007;39(1):8–13
- Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NFkappaB- dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23(1):2369–2380
- Arsenijevic D, Onuma H, Pecqueur C, et al. Disruption of then uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26(4):435–439
- Hallows WC, Lee S, Denu JM. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci USA. 2006;103(27):10230–10235
- Ahn BH, Kim HS, Song S, et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA. 2008;105(38):14447–14452
- Kong X, Wang R, Xue Y, et al. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 2010;5(7):11707
- Jung YJ, Lee JE, Lee AS, et al. SIRT1 overexpression decreases cisplatin-induced acetylation of NF-κB p65 subunit and cytotoxicity in renal proximal tubule cells. Biochem Biophys Res Commun. 2012;419(2):206–210
