Abstract
Background: The purpose of this study was to investigate the cancer incidence in patients with end-stage aristolochic acid nephropathy (AAN). Methods: A total of 102 patients with end-stage AAN treated in our hospital between 2004 and 2013 were included in this study. The correlation of cancer incidence with age, gender, dosage of aristolochic acid (AA), the type of renal replacement therapies, and the polymorphisms of quinone oxidoreductase 1 (NQO1) C609T and cytochrome P450 1A1 (CYP1A1) A4889G was examined. Results: The cancer incidence rate in our patients was 41.2% (42 in 102) including 39 cases of urinary cancer. The mortality rate in the patients with cancer was significantly higher than that in the patients without cancer (31%, 13/42 vs. 11.7%, 7/60, p < 0.05). Thirteen patients developed cancer before entering end-stage renal disease (ESRD). Cancer incidence was significantly associated with the dosage of AA consumption (p = 0.091). Hemodialysis, peritoneal dialysis and renal transplant did not affect the cancer incidence in our patients differently, but appeared to be associated with cancer at particular locations of urinary system. The patients undergoing hemodialysis seemed to more likely have bladder cancer (72.72%), while the patients receiving peritoneal dialysis appeared to develop cancer predominantly in the upper urinary tract (66.67%). Conclusions: The cancer initiation in our patients seems significantly correlate with the dosage of AA consumption. Different renal replacement therapies appear to be associated with cancer at particular locations of urinary system in our patients.
Introduction
The carcinogenic activity of aristolochic acid (AA), a component of all Aristolochia plants, was first identified in the Belgian women who took Aristolochia herbs as a weight-loss regimen and developed AA-associated end-stage renal diseases (ESRD).Citation1,Citation2 Since then, AA has been increasingly recognized as a potent nephrotoxin and human carcinogen to induce urothelial carcinoma.Citation3,Citation4 The mechanism underlying the AA-associated carcinogenesis has been clarified recently. AA damages DNA by forming covalent aristolactam (AL)-DNA adducts after metabolic activation, leading to loss-of-function mutations in tumor-suppressor geneTP53 and gain-of-function mutations in oncogenes FGFR3 and H-RAS.Citation5–9 AL-DNA adducts persist for years in the renal cortex, serving as a robust biomarker for exposure to AA.Citation10,Citation11
The prevalence of AA-associated kidney diseases, which is also called aristolochic acid nephropathy (AAN), is particularly high in Asian countries where Aristolochia herbal remedies have been used widely for many years. Taiwan has the highest incidence of AAN in the world.Citation12,Citation13 Lai et al.Citation12 conducted a population-based case-control study and found that consumption of AA-containing herbal products is associated with an increased risk of urinary tract cancer in a dose-dependent manner. In 2004, Xu et al.Citation14 from our hospital reported that the wide use of Chinese herbal medicine “Gan Lu Xiao Du Wan” (containing Mu Tong mainly), which contains substantial amounts of AA, leads to high incidence of AAN in Wenzhou China. These AAN patients have been treated in our hospital since their diagnosis. Most of them already entered ESRD and have been undergoing renal replacement therapies including hemodialysis, peritoneal dialysis and renal transplant. Some of these patients developed urinary cancer. The purpose of this study was to examine the cancer incidence and management in these patients and retrospectively analyze the correlation of cancer incidence with age, gender, the dosage of AA, type and duration of renal replacement therapy, and the polymorphisms of quinone oxidoreductase gene NQO1 and cytochrome P450 gene CYP1A1 in the patients with end-stage AAN from Wenzhou China.
Subjects and methods
Patients
The study was approved by the Institutional Ethics Committee of the First Affiliated Hospital of Wenzhou Medical College. All the patients or patients’ families signed the informed consent form. Subjects of this study included 102 patients who were diagnosed with end-stage AAN and have been undergoing renal replacement therapies in the Department of Nephrology of the First Affiliated Hospital of Wenzhou Medical College between 2004 and 2013. There were 36 cases of hemodialysis, 13 cases of peritoneal dialysis and 53 cases of renal transplants (three cases received hemodialysis due to renal failure after transplant). There were 20 cases of death among the study subjects.
The inclusion criteria for patients were: (1) took high-dose of AA-containing herbal medicine before development of AAN; (2) presented with nocturia, renal glucosuria, tubular proteinuria, low urine osmolality, anemia, progressive renal tubule damage, chronic interstitial nephritis and widowed cell renal interstitial fibrosis. The AA containing Chinese herbal medicines consumed by the patients were “Gan Lu Xiao Du Wan”, “Pai Shi Tang”, “Mu Tong” and “Long Dan Xie Gan Wan”. Patients with renal dysfunction due to the following conditions were excluded from the study: primary glomerulonephritis, autoimmune diseases, infections, hypertension and medication-induced tubulointerstitial lesions (such as antibiotics, antipyretic analgesics or chemotherapeutic drugs). Malignancy in urinary system was diagnosed by ultrasound and magnetic resonance imaging (MRI). The diagnosis was further confirmed by biopsy of tumor lesion or cystoscopy.
Polymorphism analysis
Genomic DNA was extracted by using the QIAamp DNA Blood Mini Kit (QIAGEN, Shanghai, China) from the whole blood samples collected from 66 patients. NQO1 and CYP1A1 polymorphic variants were genotyped by PCR according to the description by Atsuta et al.Citation15 The GAPDH gene was used as the reference gene. The genotype was confirmed by DNA sequencing.
Statistical analysis
Data were analyzed by the statistical analyzing software SPSS 13.0 (Hong Kong, China). Continuous variables are presented as mean ± standard deviation (SD), and categorical variables as the number of patients in each group. Comparison between two groups was analyzed by Student’s t-test. The correlation of cancer incidence with the NQO1 polymorphism 609 or CYP1A1 polymorphism 4889 was analyzed by the chi-square test. The correlation of the incidence of urinary tract cancer with gender, age, AA dosage, the type, or duration of renal replacement therapies was analyzed using multivariable logistic regression models. p < 0.1 was considered statistically significant. Fisher’s exact test was performed to compare the proportion of cancers at different locations of urinary system in patients receiving hemodialysis versus peritoneal dialysis.
Results
The mortality rate in AAN patients with cancer is significantly higher than that in AAN patients without cancer
Among the 102 subjects, 42 (41.2%) patients developed cancer and 39 cases had cancers in urinary system (). The rate of relapse in patients with bladder cancer was 37.9% (11/29, ). The rate of metastasis in patients with cancers in urinary system was 20.5% (8/39). The overall mortality rate in the 102 subjects was 19.61% (20/102). The mortality rate in the AAN patients with cancer was significantly higher than that in AAN patients without cancer (31%, 13/42 vs. 11.67%, 7/60, p < 0.05).
Table 1. The type, management and status of cancer in the patients.
Different renal replacement therapies seem to be associated with cancer at specific locations of urinary system in the AAN patients
We examined the cancer incidence and the clinical course of renal dysfunction in our patients and found that 13 patients developed cancer before entering ESRD or even before exhibiting any symptoms of renal dysfunction, and 29 patients were diagnosed with cancer 1–13 years (4.62 ± 3.31 y) after they entered ESRD and underwent renal replacement therapies (15 cases of renal transplant, 11 cases of hemodialysis and 3 cases of peritoneal dialysis). We then focused on these 29 patients and analyzed possible correlations between different renal replacement therapies and cancer incidence. Our results show that the cancer incidence in patients undergoing renal transplant, hemodialysis and peritoneal dialysis was 28.30% (15/53), 30.56% (11/36) and 23.08% (3/13), respectively. Thus, different renal replacement therapies did not seem to influence the cancer incidence in the AAN patients differently (); instead, they appeared to be associated with cancer at specific locations of urinary system. Majority of the patients (72.72%) undergoing hemodialysis had bladder cancer, while 66.67% of patients with peritoneal dialysis developed upper urinary tract cancer. In contrast, renal transplant was not associated with cancer at any particular locations of urinary system although there were slightly more cases of combining bladder and upper urinary tract cancer (40%) than cancer at either bladder or upper urinary tract ().
Figure 1. Different renal replacement therapies seemed to be associated with cancer at specific locations of urinary system. Patient undergoing hemodialysis developed cancer mainly in bladder (72.72%), while patient receiving peritoneal dialysis had cancer predominantly in upper urinary tract (66.67%). Fisher’s exact test was performed to compare the proportion of cancers at different locations of urinary system in patients receiving hemodialysis versus peritoneal dialysis. p = 0.022. Renal transplant did not appear to be associated with cancer at any particular locations of urinary system.
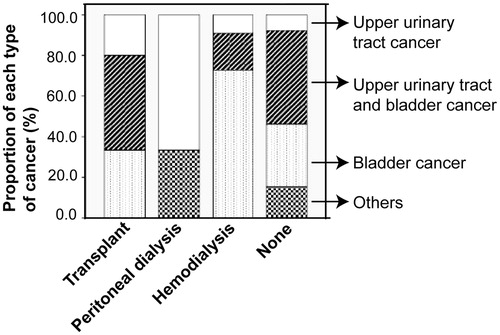
Table 2. Analysis of potential risk factors for cancer incidence in the AAN patients.
Dosage of AA consumption is the only significant risk factor for cancer incidence in AAN patients
We also analyzed the correlation between cancer incidence and gender, age, duration of renal replacement therapy, and AA dosage (Mu Tong consumption). AA dosage was the only significant risk factor for cancer incidence (p = 0.091, ). Compared with the patients consuming less than 60 g of Mu Tong, relative risk of cancer incidence increased 1.70-folds (p = 0.544, 95% CI: 0.3–9.3) in the patients consuming 60–200 g of Mu Tong and increased 4.26-folds (p = 0.048, 95% CI: 1.02–17.83) in the patients consuming more than 200 g of Mu Tong. All the other factors did not significantly correlate with the cancer incidence in the patients. We also examined the correlation of cancer incidence with the polymorphisms of NQO1 C609T and CYP1A1 A4889G in 66 patients. Our results show that the polymorphisms were not associated with cancer incidence in our patients ().
Table 3. Analysis of correlation between polymorphism and cancer incidence in the AAN patients.
Discussion
Our study demonstrates that the incidence rate of cancer in our patients was 41.2%, which is similar to what have been reported by others.Citation16,Citation17 Nortier et al.Citation16 found that the prevalence of urothelial carcinoma in 39 patients with end-stage AAN and undergoing prophylactic surgery was 46%. Cosyns et al.Citation17 showed that urothelial carcinoma was present in 4 out of 10 patients (40%) with AAN. In our study, 13 patients who consumed more than 60 g of Mu Tong developed cancer before entering ESRD or exhibiting any symptoms of renal dysfunction, further confirming the carcinogenicity of AA. Nortier et al.Citation18 also presented one case of invasive urothelial cancer after exposure to AA without significant renal failure. Soloway et al. found urothelial cancer to be highly metastatic. Despite aggressive treatment approximately 25% of patients with superficial bladder cancer will develop metastasis.Citation19,Citation20 Similarly, the metastasis rate in our patients with AAN was 20%. Chen et al.Citation21 compared the clinical and pathological characteristics of AA-induced versus non-AA-induced upper urinary tract urothelial cancer and found that patients with AA-induced cancer had a significantly higher risk of contralateral recurrence compared with patients with non-AA-induced cancer.
Consistent with the results from the study by Lai et al.,Citation12 which shows that the prescribed dosage of Mu Tong or the estimated cumulative dose of AA is significantly associated with the risk of urinary tract cancer, we also found that patients consuming high dosage of AA had a significantly increased risk of urinary tract cancer. The 13 patients who developed cancer before entering ESRD, all consumed more than 60 g of Mu Tong. Although we did not analyze the level of AA-induced DNA adducts in the patients consuming different dosage of AA, a strong linear correlation between the dosage of AA exposure and the level of AA-associated DNA adducts have been observed in rats.Citation22,Citation23 In addition, Mei et al.Citation22 demonstrated that a significant increase in AA-induced DNA adduct formation is associated with high incidence of kidney tumor in rats. Chen et al.Citation23 also found that a strong linear relationship between mutation frequency in the kidney of rats and the treatment dosage of AA, and the mutation frequency correlates with tumor incidence in the kidney.
NQO1 and CYP1A1 have been identified as the most important enzymes activating AA I to generate reactive intermediate cyclic nitrenium ion. This electrophilic intermediate subsequently reacts with DNA to form DNA adducts, leading to genetic mutations and tumorigenesis. Stiborova et al.Citation24 suggested that one cause for cancer development in some but not all AAN patients may be individual differences in the activities of the enzymes involved in AA activation, and different enzyme activity may be associated with polymorphisms in the genes of those enzymes. NQO1 C609T polymorphism (the genotype NQO1*2/*2) appears to predispose patients suffering from Balkan Endemic Nephropathy (BEN) to the development of urothelial malignancy.Citation25 Contrarily, we did not find any correlation between the NQO1 polymorphism and cancer incidence in our patients. CYP1A1 A4889G polymorphism has been shown to be associated with less aggressive breast cancerCitation26; while in our study, it did not correlate with the cancer incidence in our patients.
Different types of renal replacement therapies have been found to influence cancer risk in patients with ESRD. Chung et al.Citation27 compared the cancer risk between newly diagnosed ESRD patients and control population matched for age, sex, index month and index year. They found that patients undergoing hemodialysis or peritoneal dialysis had 2-folds increase in cancer risk, while 4-folds increase was observed in patients receiving renal transplant.Citation27 Our study shows no significant difference in cancer incidence in patients undergoing hemodialysis, peritoneal dialysis or renal transplant. The discrepancy might be associated with the predominant carcinogenic effect of AA that masks the impact of renal replacement therapies on cancer risk in our patients. Interestingly, we found that different renal replacement therapies were associated with cancer at different locations of the urinary system. The patients undergoing hemodialysis showed high incidence of bladder cancer, while those undergoing peritoneal dialysis seemed more likely to develop cancer in the upper urinary tract. The high proportion of bladder cancer in patients receiving hemodialysis might be associated with the decrease in urine output. The reduction of urine output reduces the washing and flushing on the bladder substantially and consequently increases the “dwelling time” in the bladder of the urothelial cancer cells shed from the upper urinary tract, consequently increasing the chance of implantation of those cancer cells in the bladder and causing bladder cancer. The number of patients in our study is small. We are currently collaborating with hospitals in other regions of China to enroll patients with end-stage AAN and further investigate the characteristics of cancer initiation and development associated with AAN.
Conclusions
Our study demonstrated that the incidence rate of cancer in our patients with end-stage AAN was 41.2%. High dosage of AA significantly increased cancer risk in AAN patients. We also found that our patients undergoing hemodialysis seemed more likely to develop bladder cancer, while those receiving peritoneal dialysis appeared to have cancer predominantly in upper urinary tract.
Declaration of interest
The authors declare that they have no competing interests.
This work was supported by research grants (Dr Feifei Xu) from the Health Department of Zhejiang Province.
Notes
* Part of the data has been published in “Chinese Journal of Nephrology” in Chinese.
References
- Vanherweghem JL, Depierreux M, Tielemans C, et al. Rapidly progressive interstitial renal fibrosis in young women: Association with slimming regimen including Chinese herbs. Lancet. 1993;13:387–391
- Vanhaelen M, Vanhaelen-Fastre R, But P, Vanherweghem JL. Identification of aristolochic acid in Chinese herbs [letter]. Lancet. 1994;343:174
- Schmeiser HH, Kucab JE, Arlt VM, et al. Evidence of exposure to aristolochic acid in patients with urothelial cancer from a Balkan endemic nephropathy region of Romania. Environ Mol Mutagen. 2012;53:636–641
- Bui-Klimke T, Wu F. Evaluating weight of evidence in the mystery of Balkan endemic nephropathy. Risk Anal. 2014;34:1688–1705
- Arlt VM, Stiborova M, Schmeiser HH. Aristolochic acid as a probable human cancer hazard in herbal remedies: A review. Mutagenesis. 2002;17:265–277
- Cosyns JP, Jadoul M, Squifflet JP, Wese FX, van Ypersele de Strihou C. Urothelial lesions in Chinese-herb nephropathy. Am J Kidney Dis. 1999;33:1011–1017
- Moriya M, Slade N, Brdar B, et al. TP53 Mutational signature for aristolochic acid: An environmental carcinogen. Int J Cancer. 2011;129:1532–1536
- Chen CH, Dickman KG, Moriya M, et al. Aristolochic acid-associated urothelial cancer in Taiwan. Proc Natl Acad Sci USA. 2012;109:8241–8246
- Aydin S, Dekairelle AF, Ambroise J, et al. Unambiguous detection of multiple TP53 gene mutations in AAN-associated urothelial cancer in Belgium using laser capture microdissection. PLoS One. 2014;9:e106301
- Jelakovic B, Karanović S, Vuković-Lela I, et al. Aristolactam-DNA adducts are a biomarker of environmental exposure to aristolochic acid. Kidney Int. 2012;81:559–567
- Schmeiser HH, Nortier JL, Singh R, et al. Exceptionally long-term persistence of DNA adducts formed by carcinogenic aristolochic acid I in renal tissue from patients with aristolochic acid nephropathy. Int J Cancer. 2014;135:502–507
- Lai MN, Wang SM, Chen PC, Chen YY, Wang JD. Population-based case-control study of Chinese herbal products containing aristolochic acid and urinary tract cancer risk. J Natl Cancer Inst. 2010;102:179–186
- Lai MN, Lai JN, Chen PC, Hsieh SC, Hu FC, Wang JD. Risks of kidney failure associated with consumption of herbal products containing Mu Tong or Fangchi: A population-based case-control study. Am J Kidney Dis. 2010;55:507–518
- Xu F, Lin F, Lv Y. The correlation between Gan Lu Xiao Du Wan and the incidence of tubulointerstitial nephritis and urinary tract tumor. Chinese J Nephrol. 2004;20:21–23
- Atsuta Y, Kawase H, Hamajima N, et al. Use of duplex PCR-CTPP methods for CYP2E1RsaI/IL-2 T-330G and IL-1B C-31T/TNF-A T-1031C polymorphisms. Mol Diagn. 2005;9:89–94
- Nortier JL, Martinez MC, Schmeiser HH, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N Engl J Med. 2000;342:1686–1692
- Cosyns J-P, Jadoul M, Squifflet J-P, Wese F-X, van Ypersele de Strihou C. Urothelial lesions in Chinese-herb nephropathy. Am J Kidney Dis. 1999;33:1011–1017
- Nortier JL, Schmeiser HH, Muniz Martinez MC, et al. Invasive urothelial carcinoma after exposure to Chinese herbal medicine containing aristolochic acid may occur without severe renal failure. Nephrol Dial Transplant. 2003;18:426–428
- Vishnu P, Mathew J, Tan WW. Current therapeutic strategies for invasive and metastatic bladder cancer. Onco Targets Ther. 2011;4:97–113
- Soloway MS, Sofer M, Vaidya A. Contemporary management of stage T1 transitional cell carcinoma of the bladder. J Urol. 2002;167:1573–1583
- Chen CH, Dickman KG, Huang CY, et al. Aristolochic acid-induced upper tract urothelial carcinoma in Taiwan: Clinical characteristics and outcomes. Int J Cancer. 2013;133:14–20
- Mei N, Arlt VM, Phillips DH, Heflich RH, Chen T. DNA adduct formation and mutation induction by aristolochic acid in rat kidney and liver. Mutat Res. 2006;602:83–91
- Chen L, Mei N, Yao L, Chen T. Mutations induced by carcinogenic doses of aristolochic acid in kidney of Big Blue transgenic rats. Toxicol Lett. 2006;165:250–256
- Stiborova M, Frei E, Schmeiser H. Biotransformation enzymes in development of renal injury and urothelial cancer caused by aristolochic acid. Kidney Int. 2008;73:1209–1211
- Toncheva DI, Von Ahsen N, Atanasova SY, Dimitrov TG, Armstrong VW, Oellerich M. Identification of NQO1 and GSTs genotype frequencies in Bulgarian patients with Balkan endemic nephropathy. J Nephrol. 2004;17:384–389
- Cardoso-Filho C, Sarian LO, de Oliveira CB, et al. Clinical effects of A4889G and T6235C polymorphisms in cytochrome P-450 CYP1A1 for breast cancer patients treated with tamoxifen: Implications for tumor aggressiveness and patient survival. Cancer Chemother Pharmacol. 2013;72:529–535
- Chung CJ, Huang CY, Tsai HB, et al. Sex differences in the development of malignancies among end-stage renal disease patients: A nationwide population-based follow-up study in Taiwan. PLoS One. 2012;7:e44675
