Abstract
Background: Diabetic nephropathy is the most common cause of end-stage renal disease. Emerging evidences indicate that many mechanistic pathways including apoptosis play an important role in the pathogenesis and progression of macrovascular and microvascular complications of diabetes mellitus. The aim of the present study is to show the effects of grape seed extract (GSE) on oxidative stress and apoptosis in the kidney of streptozotocin-induced diabetic rats. Materials and methods: The study included control group, diabetic group without treatment and diabetic group treated with GSE (n = 7) group. GSE was given orally (100 mg/kg/day) for six weeks. Following parameters were evaluated; oxidative stress index, caspase 1, IL1-alpha, caspase 2, IL1-beta, BCL2-associated agonist of cell death (BAD), X-linked inhibitor of apoptosis (XIAP), DNA fragmentation factor, alpha subunit and beta bubunit (DFFA, DFFB), BH3 interacting domain death agonist (BID), caspase 6, Bcl2-like 1 (BCL-XL), caspase 8, tumor necrosis factor receptor superfamily, member 1 b (TNFRSF1B) and IAP-binding mitochondrial protein (DIABLO). Results: Oxidative stress index levels were significantly increased in the kidney of diabetic group without treatment compared to control group, and decreased in diabetic + GSE group compared to diabetic group without treatment. In the kidney of diabetic group without treatment, caspase 1, IL-1 alpha, BAD, DFFA, DFFB and caspase-6 gene expressions were significantly higher compared to control group. In diabetic + GSE group caspase 1, caspase 2, XIAP, DFFA, BID, BCL-XL and TNFRSF1B genes were significantly decreased compared to control group. Conclusions: Grape seed reduces oxidative stress and apoptosis gene expression suggesting the protective effect on diabetic nephropathy.
Introduction
Despite the diagnostic tools and therapeutic implications, the prevalence of diabetes mellitus (DM) is growing and diabetic kidney disease has become the most common cause of end-stage renal disease (ESRD) in the world. Based on the World Health Organization’s report, DM is a metabolic disorder of multiple etiologies characterized by chronic hyperglycemia and various metabolic disturbances resulting from defects in insulin secretion, insulin action or both.Citation1 Large-scaled researches demonstrated that hyperglycemia is one of the major contributing factor in the pathogenesis of complications associated with DM.Citation2 The glucose uptake from kidney is independent of insulin; therefore, the increased plasma glucose levels in DM result in high intracellular glucose levels in the kidney.Citation3 Chronic hyperglycemia is the main indicator in the initiation and progression of diabetic nephropathy; it not only produces reactive oxygen metabolites but also decreases the antioxidant mechanism via non-enzymatic glycosylation of antioxidant enzymes.Citation4 Although DM is traditionally viewed as a non-immune disease, emerging evidences indicate that many mechanistic pathways including apoptosis play an important role in the pathogenesis and progression of macrovascular and microvascular complications of this chronic metabolic disease.Citation5 One of the programmed cell death mechanism is apoptosis that is mainly associated with caspase system. Apoptosis is characterized by chromatin condensation, cell surface blebbing, nuclear DNA fragmentation and eventually cell shrinkage.Citation6 In vivo and in vitro studies report that high blood glucose concentration causes production of superoxide O2, nitric oxide and peroxynitrite production in kidney cells.Citation7,Citation8 Peroxynitrite is a free radical shown to activate various caspases including caspase 3 that plays a fundamental role in inducing apoptosis in HL-60 leukemic cells.Citation9
Previous studies frequently used diabetic animal models induced with streptozotocin (STZ) for DM-associated renal damage development. In the progression of diabetic nephropathy, besides glomerular damage, tubulointerstitial fibrosis is also considered as an important pathological process.Citation10 Both glomerular cells including podocytes, mesangial cells and tubulointerstitial cells lay out apoptosis that is also associated with the progression of diabetic nephropathy.Citation5 Islet beta cell apoptosis occurs especially in the form of immune-mediated (Type 1) DM.Citation11 These models demonstrated that long-term hyperglycemia impairs renal structure and function.
The strong antioxidant features of grape seed are well known.Citation14,Citation15 There are studies in the literature demonstrating nephroprotective effects of grape seed in diabetic nephropathy, however, the underlying mechanisms of these effects are yet to be explained.Citation12 Since therapeutic targets against the apoptosis are very important to prevent the chronic complications including nephropathy, neuropathy and vasculopathy of diabetes, we hypothesized that grape seed can prevent the apoptosis and oxidant status. Hence, in the present study, we aimed to investigate the nephroprotective effects of grape seed in the experimental model of STZ-induced DM in rats.
Materials and methods
Animal and experimental design
After the necessary ethics committee approval was obtained, the animals for the study were provided from Experimental Animals Laboratory of Medical School of Pamukkale University, Denizli, Turkey. For this study, 28 adult male Wistar rats weighing 250–300 g were used. The rats were randomly assigned to three equivalent groups (n = 7).
Group 1 (n = 7): Control group
Group 2 (n = 7): Induced DM with 60 mg/kg STZ, administered 0.5 mL saline by oral gavage for 8 weeks.
Group 3 (n = 7): Induced DM with 60 mg/kg STZ, administered 100 mg/kg/day grape seed (dissolved in 0.5 mL saline) by oral gavage for 8 weeks.
Throughout the study, all the rats were kept in laboratory conditions at room temperature (22 ± 2 °C), with 50 ± 5% humidity rate, and with a 12-h light–dark cycle, in special cages with dimensions of 30 × 35 × 17 cm that have plastic bottoms and wire tops. They were fed with the same type of baits without nutritional limitations.
Drugs
STZ and α-tocopherol (vitamin E) were obtained from Sigma (St. Louis, MO). Grape seed extracts (GSE) were obtained from the wastes of grape molasses production (Department of Food Engineering, Pamukkale University, Denizli, Turkey). Manually cleaned seeds were ground with an electric coffee mill (MKM 6000, Type KM 13, Bosch Inc., Istanbul, Turkey). Powdered grape seeds were mixed with aqueous ethanol (70%, v/v) at a ratio of 1:10 (w/v) and the mixture was subjected to 15 min of sonication in an ultrasonic bath. Then, it was stirred on a mechanical shaker at room temperature for 30 min. After this step, the mixture was centrifuged at 4 °C for 20 min, 8500 rpm/min. Supernatants were collected with Pasteur pipettes and transferred into 30 mL amber glass bottles. Aqueous ethanol (70%, v/v) was added to the remaining residue at a ratio of 1:10 (w/v) and extraction procedure was repeated. After volume was adjusted in volumetric flasks, pooled supernatants were stored in a freezer at −20 °C in amber glass vials before freeze drying. Before lyophilization, ethanol in supernatants was first evaporated by a rotary evaporator (Büchi, Rotavapor, Germany) under vacuum at 40 °C (∼10 min). Viscous extracts were frozen at −70 °C, and then lyophilized (Thermo M230, Germany). Freeze-dried powder was collected carefully with a micro-spatula, and amber vials of lyophilized GSE were stored at −24 °C until analyses. Experiments were followed gravimetrically and the extraction efficiency was calculated at the end of the process. Antioxidant activity was evaluated using the Ferric Reducing Ability of Plasma (FRAP) method. Gallic acid, catechin and epicatechin contents of GSE were determined. Materials used for analyses are given in .
Table 1. Extraction properties of the freeze-dried GSEs and the efficiency of the extraction procedure.
Table 2. Phenolic content (on a dry matter basis) and antioxidant activity (FRAP method) of grape seeds obtained from the wastes of grape molasses production.
Table 3 Gallic acid, catechin and epicatechin contents of freeze-dried extracts of grape seeds.
Induction of experimental DM with STZ in rats
In order to induce experimental DM, rats allocated for DM groups were injected 60 mg/kg STZ (Sigma, Germany) dissolved in 0.5 mL saline (0.9% NaCL) intraperitoneally (IP). On day 3 following the STZ administration, the glucose levels of the rats were measured using blood obtained from their tail vein. Rats with serum glucose over 300 mg/dL were considered diabetic.Citation13–14 The study time line and associated applications are presented in .
Table 4. Experimental groups and study flowchart.
All rats were weighted first and then sacrificed 8 weeks after DM induction via decapitation under general anesthesia provided by i.p. injection of 90 mg/kg ketamine hydrochloride (Ketalar, Parke-Davis, Istanbul, Turkey) and 10 mg/kg of 2% xylazine hydrochloride (Rompun, Bayer, Istanbul, Turkey) combination. Afterwards, the rats’ kidney tissues were removed.
Assessment of oxidant/antioxidant status
Kidney tissue samples were homogenized and the supernatant of the homogenate obtained by centrifugation at 12,000 rpm for 15 min at +4 °C was used for measurements. The tissue protein concentration was measured using the Lowry method. Total oxidant status (TOS) of tissue samples was determined by an automated colorimetric method.Citation15 Briefly, an oxidation reaction is developed by glycerol molecules in the reaction medium and oxidants in the sample are left to oxidize ferrous ion-O-dianisidine complex to ferric ion. The ferric ion forms a colored complex with xylenol orange in an acidic medium that can be measured via spectrophotometry to estimate the total amount of oxidant molecules in the tissue sample. The assay is calibrated with hydrogen peroxide and the results are expressed as hydrogen peroxide equivalent per milligram protein (μmol H2O2 Eq/mg protein).
Total antioxidant status (TAS) in the tissue samples was also determined by an automated colorimetric method.Citation16 Hydroxyl radical produced by the Fenton reaction reacts with the colorless substrate O-dianisidine to produce the dianisyl radical, which is bright yellowish-brown in color. When plasma is added, the oxidative reactions initiated by the hydroxyl radicals are suppressed by the antioxidant components in the plasma so that the color change is prevented providing an effective measurement of TAS, with precision lower than 3%. The assay results are expressed as mmol Trolox Eq/mg protein.
The ratio of TOS to TAS is defined as oxidative stability index (OSI) according to the following formula:
RNA isolation
Kidney tissues were divided into small pieces in TRIzol solution on ice. Total RNA was extracted by using an RNA isolation reagent (TRI Reagent) procedure (Sigma, St. Louis, MO). Complementary DNA generation was induced by EasyScript Plus cDNA kit.
Relative quantification of apoptosis gene expression
Real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR) analyses of all genes were performed for 96 well micro plates at StepOne Plus RT PCR instrument and software. Beta actin housekeeping gene was the standard to control the variability in amplification. RT-PCR was performed by using EvaGreen quantitative polymerase chain reaction (qPCR) master mix-ROX kit according to the instructions of the manufacturer. The experiments were repeated twice with duplicates in each group.
Blood urea levels were measured via urease method by BS 300 automated biochemical device, and creatinine via creatinine Jaffa method by BS 300 non-automatized biochemical device.
Statistical analysis
The findings were analyzed with the Delta CT method and quantitated with Light Cycler Quantification Software. The statistical comparisons were performed using Student’s t-test. Parametric and non-parametric tests of cases and controls have been evaluated with the SPSS 15.0 (Chicago, IL).
Results
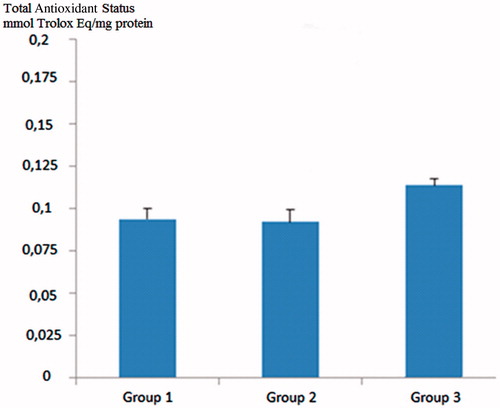
Total oxidant status value was found to be high in diabetic rats compared to control rats. In grape seed-treated diabetic rats, the TOS values were statistically significantly lower than the diabetic rats (p < 0.05) (). Total antioxidant status of the GSE-administered group was also higher than both the control group and the diabetic rats, but the difference was not statistically significant (p > 0.05) ().
Figure 1. Comparison of total oxidative status (TOS) values across groups. Group 1; control group, Group 2; diabetic rats, Group 3; grape seed treated diabetic rats. *Group 2 versus Group 3; p < 0.05.
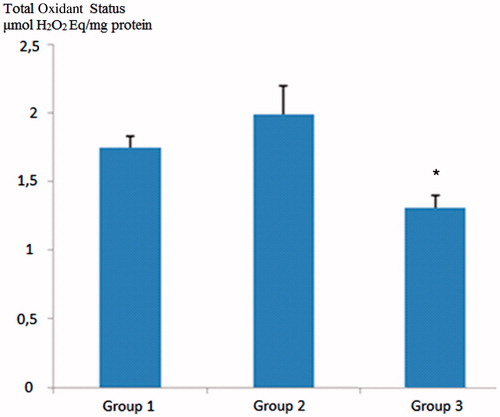
Figure 2. Comparison of total antioxidant status (TAS) values across groups. Group 1; control group, Group 2; diabetic rats, Group 3; grape seed treated diabetic rats. Group 1 versus Group 3; p: NS, Group 2 versus Group 3; p: NS.
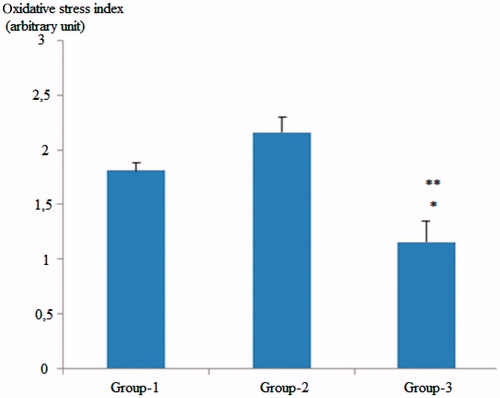
Regarding the OSI values of the groups, the values observed in the GSE-treated group were significantly lower than the OSI values of both the control and the diabetic groups (p < 0.01 for control group and p < 0.001, for the diabetic rat group) ().
Figure 3. Comparison of oxidative stability index (OSI) values across groups. Group 1; control group, Group 2; diabetic rats, Group 3; grape seed-treated diabetic rats. *Group 1 versus Group 3; p < 0.001. **Group 2 versus Group 3; p < 0.001.
We found that caspase 1 (CASP1), interleukin 1 alpha (IL1-alpha), BCL2-associated agonist of cell death (BAD), DNA fragmentation factor, alpha subunit (DFFA), DNA fragmentation factor, beta polypeptide (caspase-activated DNase) (DFFB), caspase 6 (CASP6) and Diablo IAP-binding mitochondrial protein (DIABLO) gene expression changes were significantly increased in diabetic rats compared to control rats. On the other hand, CASP1, caspase 2 (CASP2), X-linked inhibitor of apoptosis (XIAP), DNA fragmentation factor, alpha subunit (DFFA), BH3 interacting domain death agonist (BID), tumor necrosis factor receptor superfamily, Bcl2-like 1 (BCL-XL) and member 1 a (TNFRSF1B) gene expression changes were significantly decreased in grape seed-treated diabetic rats (p < 0.05) ().
Table 5. Comparison of gene expression of all groups with the control group.
We also found that BAD, DFFA, DFFB, caspase 6 and DIABLO were significantly decreased in grape seed treated rats compared to diabetic rats (p < 0.05) ().
Table 6. Comparison of gene expression of grape seed group with the diabetic group.
Discussion
Renal oxidative stress is a major contributing factor in the development of diabetic nephropathy.Citation17,Citation18,Citation22 High serum glucose levels promote the oxidative stress within the tissues via increasing the reactive oxygen radicals (ROS) through mitochondrial electron transfer chain.Citation19 ROS are highly toxic to cells, the pathogenesis of this system has been shown to increase lipid peroxidation and nuclear factor kappa β along with transforming growth factor β that causes tissue fibrosis and extracellular matrix production.Citation21 Due to high kidney blood flow, oxidant status and antioxidant enzymes play an important role in the oxidative damage in the renal tissue. In the present study, we demonstrated that the TOS value, an indicator of oxidant status, was lower in the grape seed-treated diabetic rats compared to the diabetic rats. Although the increase in TAS was not statistically significant, grape seed-treated diabetic rats had higher TAS values compared to the control group and the diabetic rats. OSI value, which indicates the oxidant status/antioxidant status ratio, was significantly lower in the grape seed-treated diabetic rats compared to the control group and the DM group. Ulusoy et al. have shown that grape seed increased TAS level and decreased TOS level in cyclosporine A-induced nephropathy.Citation20
In accordance with the findings of our study, Li et al.Citation23 reported antioxidant features of grape seed in STZ-induced diabetic rats. Grape seed proanthocyanidin extract (GSPE) was shown to increase glutathione levels in pancreatic tissue of diabetic rats, resulting in decreased lipid peroxidation, and increased insulin sensitivity by directly affecting the insulin signaling pathways.Citation24 Bagchi et al. also demonstrated that GSPE provides significant levels of protection against ROS and the free radicals that induce lipid peroxidation and DNA damage.Citation25 In another experimental study, GSPE was determined to have antioxidative and nephroprotective effect via inhibition of AGE formation.Citation12
Apoptosis is defined as programmed cell death. Although apoptosis is a natural phenomenon in all of the multi-cellular organisms, the accelerated apoptosis of endothelial cells plays a vital role in the development of diabetic vascular complications.Citation26 Kumar et al. have shown that apoptotic cell death plays a role in the occurrence of kidney damage in diabetic nephropathy.Citation27 Wong et al. reported that elevated blood glucose levels affect apoptosis-controlling genes.Citation28
We found that CASP1, IL1-alpha, BAD, DFFA, DFFB, CASP6 and DIABLO gene expression changes were significantly increased in diabetic rats compared to control rats. Caspases are known to be critically involved in two activities: activation of proinflammatory cytokines and the initiation and execution of apoptosis.Citation29,Citation30 Caspases affected in apoptosis can be divided further into initiators (cas-2,6,8,9,10,12) and executioners (cas-3,6,7).Citation31 A time course at different stages of diabetes has shown a distinct pattern of caspase activation and deactivation in the kidney. The activation of several initiator caspases occurred early in the course of diabetes.Citation32 Koenen et al. have shown that hyperglycemia increases the expression of caspase 1 and IL-1β.Citation33 Cohen et al. have showed that the level of caspase 6 increased in the type 2 diabetic rats as compared to the lean controls.Citation34 Moreover, Ulusoy et al. showed that the level of caspase 1 reduced in diabetes mellitus.Citation35 In line with the studies, the levels of caspase 1 and IL-1α, and IL-1β were determined to be higher in the diabetic group.
In our study, increase in the inflammatory markers such as caspase 1 and IL-1β and increase in the initiator caspase; caspase 6 in the diabetic rats may show the development of early stage of the diabetic nephropathy.
Moreover, we found significantly higher levels of Bad (BCL2-associated agonist of cell death) in diabetic rats. The Bcl-2-associated death promoter (BAD) protein is a pro-apoptotic member of the Bcl-2 gene family which is involved in initiating apoptosis.Citation36 In the literature, there is no data regarding the levels of BAD in the diabetic nephropathy.
DFF-mediated nuclear destruction constitutes an evolutionarily conserved mechanism that plays a pivotal role in the execution of apoptotic cellular death.Citation37 Smac/DIABLO is a mitochondria protein but is released into the cytosol in response to some apoptotic stimuli, such as UVB-irradiation, etoposide or glucocorticoid. Mature Smac/DIABLO was found to promote caspase activation by binding and neutralizing the IAPs, including XIAP, CIAP-1 and CIAP-2.Citation38,Citation39 Yeo et al. also reported that Diablo was elevated in diabetic mice.Citation40
In our study, CASP1, CASP2, XIAP, DFFAB, BCL-XL and TNFRSF1B gene expression changes were significantly decreased in grape seed treated diabetic rats. This is the first study in the literature reporting the suppressive effect of grape seed on the expression of apoptosis genes such as caspase 1, caspase 2, caspase 8, DFFAB, TNFRSF1B, BID, BAD and DIABLO playing role in the pathogenesis of diabetic nephropathy.
In the extrinsic pathway of apoptosis; several extrinsic ligands can activate “death receptors” of the TNFR Family, including apoptosis antigen-1, or TNFR1. The FAS ligand is a member of the TNF superfamily of cytokines and along with its receptor, it plays role in inflammatory and immune response.Citation41 FASL-mediated activation of FAS leads to caspase 8 activation.Citation42,Citation43 Several caspase 8 molecules cause auto-activation and processing of caspase 8 molecules which are then ready to activate downstream caspasesCitation44 resulting in cleavage of BID to truncated BID, a pro-apoptotic member of BCL 2 family. In our study, “Lower” levels of TNFRS 1, caspase 8 and BID in grape seed treated diabetic group may suggest that grape seed shows its activity on the extrinsic pathway of apoptosis.
In conclusion, the present study demonstrated that treatment with grape seed might be beneficial against oxidative stress and apoptosis in diabetic rats. Further comprehensive experimental and clinical studies are needed to investigate the beneficial effects of grape seed extracts in DM.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alberti KGMM, Aschner P, Benet PH. Definition, diagnosis and classification of diabetes mellitus and its complications. Report of WHO consultation. Geneva: World Health Organization; 1999: Part 1;2–3
- Calcutt NA, Cooper ME, Kern TS, Schmidt AM. Therapies for hyperglycaemia-induced diabetic complications: From animal models to clinical trials. Nat Rev Drug Discov. 2009;8:417–429
- Lachin JM, Genuth S, Cleary P. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–389
- Paolisso G, D'Amore A, Galzerano D, et al. Daily vitamin E supplements improve metabolic control but not insulin secretion in elderly type II diabetic patients. Diabetes Care. 1993;16:1433–1437
- Su J, Zhou L, Kong X, et al. Endoplasmic reticulum is at the crossroads of autophagy, inflammation, and apoptosis signaling pathways and participates in the pathogenesis of diabetes mellitus. J Diabetes Res. 2013;2013:193461
- Galluzzi L, Maiuri MC, Vitale I, et al. Cell death modalities: Classification and pathophysiological implications. Cell Death Differ. 2007;14:1237–1243
- Ishii N, Patel KP, Lane PH, et al. Nitric oxide synthesis and oxidative stress in the renal cortex of rats with diabetes mellitus. J Am Soc Nephrol. 2001;12:1630–1639
- Du X, Stocklauser-Farber K, Rosen P. Generation of reactive oxygen intermediates, activation of NF-kappaB, and induction of apoptosis in human endothelial cells by glucose: Role of nitric oxide synthase? Free Radic Biol Med. 1999;27:752–763
- Zhuang S, Simon G. Peroxynitrite-induced apoptosis involves activation of multiple caspases in HL-60 cells. Am J Physiol Cell Physiol. 2000;279:341–351
- Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20:1–17
- Tanaka S, Aida K, Nishida Y, Kobayashi T. Pathophysiological mechanisms involving aggressive islet cell destruction in fulminant type 1 diabetes. Endocr J. 2013;60:837–845
- Liu YN, Shen XN, Yao GY. Effects of grape seed proanthocyanidins extracts on experimental diabetic nephropathy in rats. Wei Sheng Yan Jiu. 2006;35:703–705
- Ibrahim HN, Hostetter TH. Diabetic nephropathy. J Am Soc Nephrol. 1997;8:487–493
- Yan HD, Li XZ, Xie JM, Li M. Effects of advanced glycation end products on renal fibrosis and oxidative stress in cultured NRK-49F cells. Chin Med J (Engl). 2007;120:787–793
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285
- Rincon J, Pedreanez A, Viera N, Arcaya JL, Carrizo E, Mosquera J. Depressive status does not alter renal oxidative and immunological parameters during early diabetic nephropathy in rats. World J Biol Psychiatry. 2009;10:560–566
- Anjaneyulu M, Chopra K. Diltiazem attenuates oxidative stress in diabetic rats. Ren Fail. 2005;27:335–344
- Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790
- Ulusoy S, Ozkan G, Yucesan FB, et al. Anti-apoptotic and anti-oxidant effects of grape seed proanthocyanidin extract in preventing cyclosporine A-induced nephropathy. Nephrology (Carlton). 2012;17:372–379
- Emrullah B, Ali BÇ, Esra E, et al. Changes of total antioxidant capacity and total oxidant status of aqueous humor in diabetes patients and correlations with diabetic retinopath. Int J Ophthalmol. 2013;6:531–536
- Anjaneyulu M, Tirkey N, Chopra K. Attenuation of cyclosporine-induced renal dysfunction by catechin: Possible antioxidant mechanism. Ren Fail. 2003;25:691–707
- Li BY, Cheng M, Gao HQ, et al. Back-regulation of six oxidative stress proteins with grape seed proanthocyanidin extracts in rat diabetic nephropathy. J Cell Biochem. 2008;104:668–679
- Pinent M, Blay M, Blade MC, Salvado MJ, Arola L, Ardevol A. Grape seed-derived procyanidins have an antihyperglycemic effect in streptozotocin-induced diabetic rats and insulinomimetic activity in insulin-sensitive cell lines. Endocrinology. 2004;145:4985–4990
- Bagchi D, Bagchi M, Stohs SJ, et al. Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicology. 2000;148:187–197
- Okouchi M, Okayama N, Aw TY. Preservation of cellular glutathione status and mitochondrial membrane potential by N-acetylcysteine and insulin sensitizers prevent carbonyl stress-induced human brain endothelial cell apoptosis. Curr Neurovasc Res. 2009;6:267–278
- Kumar D, Zimpelmann J, Robertson S, Burns KD. Tubular and interstitial cell apoptosis in the streptozotocin-diabetic rat kidney. Nephron Exp Nephrol. 2004;96:77–88
- Wong VY, Keller PM, Nuttall ME, et al. Role of caspases in human renal proximal tubular epithelial cell apoptosis. Eur J Pharm. 2001;433:135–140
- Alnemri E, Livingston DJ, Nicholson DW, et al. Human ICE/CED-3 protease nomenclature (Letter). Cell. 1996;87:171
- Alnemri ES. Mammalian cell death proteases: A family of highly conserved aspartate specific cysteine proteases. J Cell Biochem. 1997;64:33–42
- Cohen GM. Caspases: The executioners of apoptosis. Biochem J. 1997;326:1–16
- Susanne M, Xia X, Jie T, Timothy SK. Caspase activation in retinas of diabetic and galactosemic mice and diabetic patients. Diabetes. 2002;51:1172–1179
- Koenen TB, Stienstra R, van Tits LJ, et al. Hyperglycemia activates caspase-1 and TXNIP-mediated IL-1β transcription in human adipose tissue. Diabetes. 2011;60:517–524
- Cohen Z, Gonzales RF, Davis-Gorman GF, Copeland JG, McDonagh PF. Thrombin activity and platelet microparticle formation are increased in type 2 diabetic platelets: A potential correlation with caspase activation. Thromb Res. 2002;107:217–221
- Ulusoy S, Ozkan G, Mungan S, et al. GSPE is superior to NAC in the prevention of contrast-induced nephropathy: Might this superiority be related to caspase 1 and calpain 1? Life Sci. 2014;103:101–110
- Zha J, Harada H, Osipov K, Jockel J, Waksman G, Korsmeyer SJ. BH3 domain of BAD is required for heterodimerization with BCL-XL and pro-apoptotic activity. J Biol Chem. 1997;272:24101–24104
- Inohara N, Koseki T, Chen S, Wu X, Nunez G. CIDE, a novel family of cell death activators with homology to the 45 kDa subunit of the DNA fragmentation factor. EMBO J. 1998;17:2526–2533
- Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42
- Verhagen A, Ekert PG, Silke J, et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53
- Yeo SH, Noh JR, Kim YH, et al. Increased vulnerability to beta cell destruction and diabetes in mice lacking NAD(P)H: Quinone oxidoreductase 1. Toxicol Lett. 2013;219:35–41
- Hirata H, Takahashi A, Kobayashi S, et al. Caspases are activated in a branched protease cascade and control distinct downstream processin Fas-induced apoptosis. J Exp Med. 1998;187:587–600
- Rytomaa M, Martin LM, Downward J. Involvement of FADD and Caspase 8 signalling in detachment-induced apoptosis. Curr Biol. 1999;9:587–600
- Scaffidi C, Medamma JP, Krammer PH, Peter ME. FLICE is predominantly expressed as two functionally active isoforms, caspas 8/a and caspase 8/b. J Biol Chem. 1997;272:1043–1046
- Sohn D, Schulze-Osthoff K, Janicke RU. Caspase 8 can be activated by interchain proteolysis without receptor-triggered dimerization during-induced apoptosis. J Biol Chem. 2005;280:5267–5273
