Abstract
Background: In this study, we investigated the protective effect of thymol as a natural compound against cisplatin-induced nephrotoxicity by quantitative renal 99mTc-DMSA uptake and compared its effect with histopathology in mice. Materials and methods: Mice were divided into six groups as control, cisplatin (7.5 mg/kg, intraperitoneally), thymol + cisplatin (thymol; 50 and 150 mg/kg + cisplatin; 7.5 mg/kg) and thymol (50 and 150 mg/kg). Thymol was orally administrated for two days before cisplatin injection and continued for 4 days. 99mTc-DMSA was injected through the tail of mice after the drug administration. The percentage of the injected dose per gram of kidney tissue (%ID/g) was calculated. In other experiment, kidneys of treated mice were assessed for histopathology. Results: 99mTc-DMSA uptake per gram tissue of the kidneys as %ID/g was 85.27 ± 21.81, 45.55 ± 5.50, 65.02 ± 32.21 and 88.46 ± 20.46 in the control, cisplatin, thymol (50 mg/kg) + cisplatin and thymol (150 mg/kg) + cisplatin. Thymol administration with cisplatin resulted in a significant increase in the level of %ID/g. Histopathological examinations showed a protective effect of thymol against cisplatin nephrotoxicity in mice. Conclusion: The results showed that thymol significantly attenuates the cisplatin-induced nephrotoxicity in mice, and 99mTc-DMSA uptake in kidney is a suitable method for assessment of nephrotoxicity in mice.
Introduction
Cisplatin (cis-diamminedichloroplatinum II, CDDP) is a potent antineoplastic drug, which is widely used for the treatment of various cancers such as lung, head and neck, ovarian and bladder.Citation1–5 Its clinical usage is limited by major toxicity in kidney, namely nephrotoxicity, this toxicity is a dose-limiting factor in cancer therapy with cisplatin.Citation6–8 The mechanisms of cisplatin nephrotoxicity are complex and involve multiple pathways and molecules. Cisplatin is uptake by renal tubular cells and activates complex signaling pathways that lead to tubular cell injury and death. Cisplatin also induces damage in the renal vascular and leads to reduced blood flow in kidneys.Citation9,Citation10 Oxidative stress is an important factor that contributes in pathogenesis of cisplatin-induced nephrotoxicity. Reactive oxygen species (ROS) are increased in tissue under treatment with cisplatin. The ROS directly act on the cell components, including lipids, proteins and DNA, and destroys their structure. Inflammation and TNF-α-induced apoptosis also contribute in this toxicity. Cisplatin is believed to contribute cytotoxicity through interaction with DNA. Cisplatin can react with nucleophilic sites on DNA leading to inter- and intrasternal cross-links, thereby arresting DNA synthesis and replication in cells.Citation9,Citation11 Cisplatin binds to intracellular thiol groups and leads to glutathione (GSH) depletion, resulting in lipid peroxidation and eventually mitochondrial damage.Citation12 Various approaches have been identified for prevention against cisplatin-induced nephrotoxicity, which are related to above mentioned mechanisms of toxicity. Pharmacological agents against oxidative stress and inflammation are main approaches for renoprotection against cisplatin toxicity.Citation9,Citation11
Thymol is a natural monoterpene phenolic compound that is present in various plants, such as thyme (Lamiaceae) and Zataria.Citation13–15 Thymol has been reported to have anti-inflammatory activityCitation16 and protective effects against toxicity caused by oxidative stress in liver and lymphocytes.Citation17–19 The protective properties are associated to antioxidant, free radical scavenging and anti-lipid peroxidation of thymol.Citation17,Citation20
99mTc-DMSA, dimercaptosuccinic acid, is a radiopharmaceutical agent used for functional imaging of kidneys in nuclear medicine. It is filtered and bound to α1-microglobulin and accumulates in the renal proximal tubules by megalin/cubilin-mediated endocytosis of the 99mTc-DMSA protein complex.Citation21 99mTc-DMSA is highly bound to plasma proteins in the circulating blood after injection and penetrates into the glomerular filter at very low rates. 99mTc-DMSA uptake depends on the renal blood flow and proximal tubular cell membrane transport function.Citation22 99mTc-DMSA scintigraphy has been used clinically to diagnose and monitor ifosfamide and cisplatin nephrotoxicity in patients.Citation23
In this study, we investigated the effect of thymol on the protection of cisplatin-induced nephrotoxicity by using quantitative 99mTc-DMSA uptake measurements and compared its effect with histopathology in mice.
Materials and methods
Animal
Male NMRI mice weighing 25 ± 3 g were purchased from the Pasteur Institute (Amol, Iran). Mice were housed in a good condition in the university animal house and given standard mouse pellet and water ad Libitum. All of animals were kept under controlled lighting condition (light:dark, 12:12 h) and temperature (22 ± 1°C). Animal experimental and ethical issues were approved by Research Committee of Mazandaran University of Medical Sciences, Sari, Iran at 28-8-2013 (ID# 92-146).
Chemicals
Cisplatin was obtained from MYLAN (10 mg/10 mL vial; Saint-Priest, France). Corn oil was purchased from Sigma Chemical Co. (Buchs, Switzerland). Thymol was from Sinchem Chemical Co. (Korea). All other chemicals were obtained from Merck Company (Darmstadt, Germany). Thymol was dissolved in corn oil.
Experimental group and treatment schedules
Experimental animals were randomly divided into six groups (Groups 1–6). Each group contained four mice:
Group 1: control; the mice were administered daily gavages of corn oil (10 mLkg–1 bw) for 6 days.
Group 2: cisplatin; the mice were administered a single dose of cisplatin intraperitoneal (ip) injections (7.5 mg/kg bw) at two days after corn oil treatment.
Groups 3 and 4: thymol + cisplatin; mice were orally treated with thymol (50 and 150 mg/kg bw) at two days before single dose of cisplatin treatment and continued for four days.
Groups 5 and 6: mice were treated with thymol (50 and 150 mg/kg bw) for six days.
Radiopharmaceutical assessment
99mTc, as sodium pertechnetate, was eluted from a 99Mo/99mTc generator (IEOI, Tehran, Iran) just before the radiolabeling procedure. DMSA (IEOI, Tehran, Iran) was used as a freeze-dried commercial kit. 99mTc-DMSA was prepared by adding 740 MBq of 99mTc pertechnetate to 2 mL of saline to the kit. Radiolabeling was performed according to manufacturer guideline. After slowly shaking, it is kept at room temperature for 15 min. 99mTc-DMSA was injected through the tail vein at 10 MBq in 0.1 mL. After deep anesthesia, mice were killed and the kidneys and other organs were removed by dissection 2 h after the radiopharmaceutical injection. Tissues were weighed in pre-weighed containers. Radioactivity in each organ sample was counted using a gamma counter (Delshid, Iran). The activity was expressed as a percentage of the injected dose per gram of tissue (%ID/g). The %ID/g was calculated by dividing the radioactivity count per minute in each tissue by total count injected and the mass of the organ.
Histopathological assessment
The kidney tissues were fixed in 10% formalin solution and embedded in paraffin. Sections of 4 µm thickness were taken and stained with hematoxylin and eosin and assessed with a light microscope. Histopathological scoring was performed by a pathologist. Renal tubular damage was evaluated on tubules showing degeneration.
Statistical analysis
Experimental data were expressed as the mean ± standard deviation (SD). The results were compared with the control group, and statistical analysis was performed by independent t-test to determine the significance of the difference between groups. The differences were considered significant when the p value was <0.05.
Results
The percentage of the injected dose per gram of kidneys and other tissues in control, cisplatin, thymol + cisplatin and thymol groups is shown in . Cisplatin treatment resulted in a decrease in the 99mTc-DMSA uptake in kidney compared to control group. Percentage of injected dose per gram of kidneys was %85.27 ± 21.81 and %45.55 ± 5.50 for control and cisplatin treatment groups, respectively (p < 0.05) (). Thymol treatment for 6 days increased significantly the uptake of 99mTc-DMSA in cisplatin-treated mice (p < 0.05). %ID/g was %88.46 ± 20.46 for thymol group at dose 150 mg/kg that administered to mice along with cisplatin. Administration of thymol prevented cisplatin nephrotoxicity with increasing 99mTc-DMSA uptake in kidneys. No significant changes were observed in %ID/g of 99mTc-DMSA uptake in mice treated with thymol alone at dose 50 and 150 mg/kg as compared to control group.
Figure 1. The percentage of the injected 99mTc-DMSA dose per gram of kidney tissue (%ID/g) in control, cisplatin (CIS), thymol 50 mg/kg + cisplatin (THY50 + CIS), thymol 150 mg/kg + cisplatin (THY150 + CIS), thymol 50 mg/kg (THY50) and thymol 150 mg/kg (THY150) groups are shown (n = 4). *p < 0.05 comparison of control and CIS groups, #p < 0.05 comparison of THY50 + CIS and CIS, **non-significant comparison of THY150 and control.
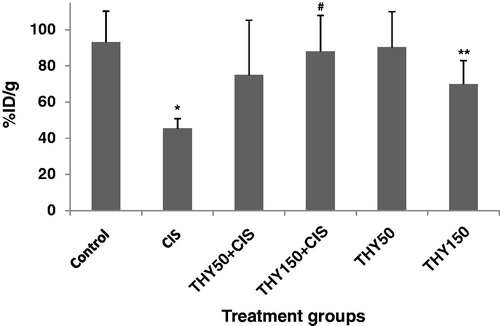
Table 1. Biodistribution of 99mTc-DMSA in mice treated with cisplatin (CIS), thymol (THY) at 2 h after injection.
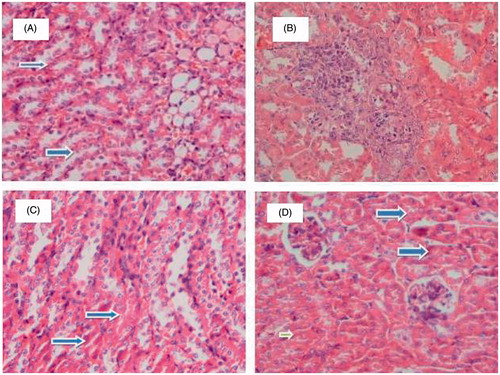
The impaired renal function caused by cisplatin was further confirmed by histological examination of kidney, from tubular degeneration (). As shown in , kidney from control mice showed no abnormality whereas kidney in animals treated with cisplatin revealed a marked tubular necrosis and degeneration, extensive epithelial vacuolization, swelling and tubular dilation and renal tubules with hyaline casts (). The tubular epithelial degeneration was less severe in the group treated with thymol + cisplatin when compared with cisplatin-treated alone mice ().
Figure 2. Histological examination of mice treated with cisplatin and/or thymol. The kidney of control mouse shows normal tubule (A). Kidney of mice administered with cisplatin at a single dose of 7.5 mg/kg exhibited a distinct histological difference when compared with control, these kidney shows large numbers of tubules with degeneration (B). Mild improvement in tubular degeneration was seen in mice treated with thymol at dose 50 mg/kg and cisplatin (C). Minimal tubular degeneration in mice treated with thymol at dose 150 mg/kg and cisplatin (D). Blue arrow shows normal tubules and green arrows shows tubular degeneration.
Discussion
Renoprotective effect of thymol after a single injection of cisplatin was evaluated in mice. Cisplatin significantly induced nephrotoxicity that is prevented by thymol. This toxicity was evaluated by using 99mTc-DMSA as a widely used radiopharmaceutical for assessment of nephrotoxicity in patients. This protective effect was confirmed with histopathology. Our results showed that determination of injected dose per gram of 99mTc-DMSA in tissue to be a good technique for assessment of nephrotoxicity and/or nephroprotective effect in the animal model. Other studies used 99mTc-DMSA for the evaluation of nephroprotective effect of carnitine, amifostine and erdosteine against cisplatin and gentamicin nephrotoxicity in animals.Citation24,Citation25 Cisplatin causes an oxidative stress in tissues. Several studies suggest that cisplatin toxicity occurs by the increased generation of ROS in tissues. Renal tissue from the cisplatin-treated animal showed decrease in the kidney endogenous antioxidant such as GSH content and superoxide dismutase (SOD) activity and also increase in malondialdehydeCitation26 production as a marker of lipid peroxidation in tissues.Citation27–29 Thus, an alteration in antioxidant status in cisplatin treatment tissues may play an important role in mitigating the free radical-induced oxidative stress on the tissue. Antioxidants have nephroprotective effects against toxicity induced by cisplatin.Citation27,Citation30 Since, thymol is a natural product that has several biological properties such as antioxidant activity and free radical scavenging, which are main mechanisms related to protective effect of thymol.Citation17,Citation20
Inflammatory process is involved in toxicity related to cisplatin. Cisplatin raised the level of several cytokines and chemokines such as interleukins, macrophage inflammatory protein and TNF-alpha, leading renal injury.Citation31,Citation32 Several pharmacological agents were reported to have nephroprotective effects against cisplatin with anti-inflammatory activity.Citation33–37 Thymol has anti-inflammatory effects and inhibited the inflammatory edema and leukocyte migration.Citation38–40 Thymol markedly inhibited the production of TNF-α and IL-6 and blocked the phosphorylation of mitogen-activated protein kinases (MAPKs) in polysaccharide-stimulated mouse mammary epithelial cells, leading to anti-inflammation in tissues.Citation41
Conclusion
The results of the present study indicated that thymol effectively protected the kidney tissue against cisplatin-induced nephrotoxicity in mice. The antioxidant and anti-inflammatory activities can be considered the main mechanisms responsible for the nephroprotective effect of thymol. Therefore, thymol may be a potential candidate to prevent renal injury which is a dose-limiting side effect during cisplatin therapy.
Declaration of interest
This research was the subject of a Pharm. D. thesis of Reza Asadian as a student of Mazandaran University of Medical Sciences. It was supported by Mazandaran University of Medical Sciences.
The authors declared no potential conflict of interest with respect to the authorship, and/or publication of this study.
SJH supervised and designed the project and prepared the manuscript; RA performed and participated in all experiments; FN and MJ performed pathological examinations; SA performed and helped in experiments; ZN, SMA and SAHH performed radiopharmaceutical experiments. All authors read and approved the final manuscript.
References
- Fruscio R, Garbi A, Parma G, et al. Randomized phase III clinical trial evaluating weekly cisplatin for advanced epithelial ovarian cancer. J Natl Cancer Inst. 2011;103(4):347–351
- Gupta S, Khan H, Barik S, Negi MP. Clinical benefits of concurrent capecitabine and cisplatin versus concurrent cisplatin and 5-flurouracil in locally advanced squamous cell head and neck cancer. Drug Discov Ther. 2013;7(1):36–42
- Stathopoulos GP, Trafalis D, Dimitroulis J, Kosmas C, Stathopoulos J, Tsavdaridis D. Combination of three cytotoxic agents in small-cell lung cancer. Cancer Chemother Pharmacol. 2013;71(2):413–418
- Wang J, Liu F, Huang DX, Jiang B. Post-operative treatment with cisplatin and vinorelbine in Chinese patients with non-small cell lung cancer: A clinical prospective analysis of 451 patients. Asian Pac J Cancer Prev. 2012;13(9):4505–4510
- Burch PA, Richardson RL, Cha SS, et al. Phase II study of paclitaxel and cisplatin for advanced urothelial cancer. J Urol. 2000;164(5):1538–1542
- Arunkumar P, Viswanatha G, Radheshyam N, Mukund H, Belliyappa M. Science behind cisplatin-induced nephrotoxicity in humans: A clinical study. Asian Pac J Trop Biomed. 2012;2(8):640–644
- Dekkers IA, Blijdorp K, Cransberg K, et al. Long-term nephrotoxicity in adult survivors of childhood cancer. Clin J Am Soc Nephrol. 2013;8(6):922–929
- Sancho-Martinez SM, Prieto-Garcia L, Prieto M, Lopez-Novoa JM, Lopez-Hernandez FJ. Subcellular targets of cisplatin cytotoxicity: An integrated view. Pharmacol Ther. 2012;136(1):35–55
- Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73(9):994–1007
- Fillastre JP, Raguenez-Viotte G. Cisplatin nephrotoxicity. Toxicol Lett. 1989;46(1–3):163–175
- Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: A review. Am J Med Sci. 2007;334(2):115–124
- Kuhlmann MK, Burkhardt G, Kohler H. Insights into potential cellular mechanisms of cisplatin nephrotoxicity and their clinical application. Nephrol Dial Transplant. 1997;12(12):2478–2480
- Sajed H, Sahebkar A, Iranshahi M. Zataria multiflora Boiss. (Shirazi thyme) – An ancient condiment with modern pharmaceutical uses. J Ethnopharmacol. 2013;145(3):686–698
- Schmidt E, Wanner J, Hiiferl M, et al. Chemical composition, olfactory analysis and antibacterial activity of Thymus vulgaris chemotypes geraniol, 4-thujanol/terpinen-4-ol, thymol and linalool cultivated in southern France. Nat Prod Commun. 2012;7(8):1095–1098
- Hosseinimehr SJ, Mahmoudzadeh A, Ahmadi A, Ashrafi SA, Shafaghati N, Hedayati N. The radioprotective effect of Zataria multiflora against genotoxicity induced by γ irradiation in human blood lymphocytes. Cancer Biother Radiopharm. 2011;26(3):325–329
- Azuma Y, Ozasa N, Ueda Y, Takagi N. Pharmacological studies on the anti-inflammatory action of phenolic compounds. J Dent Res. 1986;65(1):53–56
- Alam K, Nagi MN, Badary OA, Al-Shabanah OA, Al-Rikabi AC, Al-Bekairi AM. The protective action of thymol against carbon tetrachloride hepatotoxicity in mice. Pharmacol Res. 1999;40(2):159–163
- Archana PR, Nageshwar Rao B, Satish Rao BS. In vivo radioprotective potential of thymol, a monoterpene phenol derivative of cymene. Mutat Res. 2011;726(2):136–145
- Al-Malki AL. Antioxidant properties of thymol and butylated hydroxytoluene in carbon tetrachloride-induced mice liver injury. JKAU: Sci. 2010;22(1):239–248
- Kruk I, Michalska T, Lichszteld K, Kladna A, Aboul-Enein HY. The effect of thymol and its derivatives on reactions generating reactive oxygen species. Chemosphere. 2000;41(7):1059–1064
- Weyer K, Nielsen R, Petersen SV, Christensen EI, Rehling M, Birn H. Renal uptake of 99mTc-dimercaptosuccinic acid is dependent on normal proximal tubule receptor-mediated endocytosis. J Nucl Med. 2013;54(1):159–165
- Muller-Suur R, Gutsche HU. Tubular reabsorption of technetium-99m-DMSA. J Nucl Med. 1995;36(9):1654–1658
- Caglar M, Yaris N, Akyuz C. The utility of (99m)Tc-DMSA and Tc(99m)-EC scintigraphy for early diagnosis of ifosfamide induced nephrotoxicity. Nucl Med Commun. 2001;22(12):1325–1332
- Yurekli Y, Unak P, Yenisey C, Ertay T, Biber Muftuler FZ, Medine EI. L-carnitine protection against cisplatin nephrotoxicity in rats: Comparison with amifostin using quantitative renal Tc 99m DMSA uptake. Mol Imaging Radionucl Ther. 2011;20(1):1–6
- Cabuk M, Gurel A, Sen F, Demircan N. Renoprotective effect of erdosteine in rats against gentamicin nephrotoxicity: A comparison of 99mTc-DMSA uptake with biochemical studies. Mol Cell Biochem. 2008;308(1–2):35–42
- Yigit MV, Mazumdar D, Kim HK, Lee JH, Odintsov B, Lu Y. Smart “turn-on” magnetic resonance contrast agents based on aptamer-functionalized superparamagnetic iron oxide nanoparticles. Chembiochem. 2007;8(14):1675–1678
- Shimeda Y, Hirotani Y, Akimoto Y, et al. Protective effects of capsaicin against cisplatin-induced nephrotoxicity in rats. Biol Pharm Bull. 2005;28(9):1635–1638
- Martins NM, Santos NA, Curti C, Bianchi ML, Santos AC. Cisplatin induces mitochondrial oxidative stress with resultant energetic metabolism impairment, membrane rigidification and apoptosis in rat liver. J Appl Toxicol. 2008;28(3):337–344
- Pratibha R, Sameer R, Rataboli PV, Bhiwgade DA, Dhume CY. Enzymatic studies of cisplatin induced oxidative stress in hepatic tissue of rats. Eur J Pharmacol. 2006;532(3):290–293
- Mohan IK, Khan M, Shobha JC, et al. Protection against cisplatin-induced nephrotoxicity by Spirulina in rats. Cancer Chemother Pharmacol. 2006;58(6):802–808
- Miyawaki Y, Ueki M, Ueno M, Asaga T, Tokuda M, Shirakami G. D-allose ameliorates cisplatin-induced nephrotoxicity in mice. Tohoku J Exp Med. 2012;228(3):215–221
- Nozaki Y, Kinoshita K, Yano T, et al. Signaling through the interleukin-18 receptor alpha attenuates inflammation in cisplatin-induced acute kidney injury. Kidney Int. 2012;82(8):892–902
- Domitrovic R, Cvijanovic O, Pugel EP, Zagorac GB, Mahmutefendic H, Skoda M. Luteolin ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of platinum accumulation, inflammation and apoptosis in the kidney. Toxicology. 2013;310:115–123
- Ku CM, Lin JY. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013;141(2):1104–1113
- Liu Y, Webb HK, Fukushima H, et al. Attenuation of cisplatin-induced renal injury by inhibition of soluble epoxide hydrolase involves nuclear factor kappaB signaling. J Pharmacol Exp Ther. 2012;341(3):725–734
- Sahu BD, Kuncha M, Sindhura GJ, Sistla R. Hesperidin attenuates cisplatin-induced acute renal injury by decreasing oxidative stress, inflammation and DNA damage. Phytomedicine. 2013;20(5):453–460
- Sultana S, Verma K, Khan R. Nephroprotective efficacy of chrysin against cisplatin-induced toxicity via attenuation of oxidative stress. J Pharm Pharmacol. 2012;64(6):872–881
- Fachini-Queiroz FC, Kummer R, Estevao-Silva CF, et al. Effects of thymol and carvacrol, constituents of Thymus vulgaris L. essential oil, on the inflammatory response. Evid Based Complement Alternat Med. 2012;2012:657026
- Riella KR, Marinho RR, Santos JS, et al. Anti-inflammatory and cicatrizing activities of thymol, a monoterpene of the essential oil from Lippia gracilis, in rodents. J Ethnopharmacol. 2012;143(2):656–663
- Braga PC, Dal Sasso M, Culici M, Bianchi T, Bordoni L, Marabini L. Anti-inflammatory activity of thymol: Inhibitory effect on the release of human neutrophil elastase. Pharmacology. 2006;77(3):130–136
- Liang D, Li F, Fu Y, et al. Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-kappaB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation. 2014;37(1):214–222
