Abstract
Background: Oxidative stress and vasoconstriction appear to be important components of contrast nephropathy (CN) pathogenesis, and both carvedilol and nebivolol are known to have vasodilatory and antioxidant effects. Aims: This study aimed to investigate whether carvedilol and nebivolol play preventive roles against developing CN and to compare the effects of each. Materials and methods: Wistar albino rats were divided into control (C, n = 6), contrast material (CM, n = 6), carvedilol (CV, n = 7), carvedilol + contrast material (CV + CM, n = 7), nebivolol (N, n = 7), and nebivolol + contrast (N + CM, n = 7) groups. Following 3 days of dehydration, 6 mL/kg diatrizoate was administered to each rat. Carvedilol was given at a dose of 2 mg/kg and nebivolol at a dose of 1 mg/kg by way of oral gavage. After scarification, total antioxidant capacity (TAC), malondialdehyde (MDA), and superoxide dismutase (SOD) were studied in renal tissue. Histopathological findings were graded as mild (+), moderate (++), and severe (+++). Results and discussion: Most of the histopathological findings and MDA levels were significantly higher in the CM group than that in the C, CVCM, and NVCM groups, whereas there was no significant difference between the C, CVCM and NVCM groups. TAC level in the CM group was significantly lower than in all other groups. There was no difference in SOD among groups. Conclusions: Carvedilol and nebivolol both prevent development of nephropathy related to CMs by decreasing oxidative stress. Neither is superior to the other.
Introduction
Contrast nephropathy (CN), which develops with increased use of imaging methods that use contrast material (CM), is considered one of the most important complications of imaging procedures. Contrast-induced acute renal failure is a clinical condition that develops after exposure to iodinated CM and, generally, is known as CN.Citation1 In the literature, various values have been used to define CN, but the most commonly used definition is an increase of 0.5 mg/dL (44.2 μmol/L) or 25% in basal serum creatinine value 48 h after CM without any other reason.Citation2 The in-hospital mortality rate of CN may be as high as 20%.Citation3
Although the rate of CN is estimated to be between 1 and 2% in the general population, it may increase to 25% in the elderly, in patients with diabetes and previous renal failure, in patients who have experienced acute coronary syndrome and have undergone coronary intervention, and in patients with heart failure.
Various mechanisms have been proposed in the pathogenesis of CN, including renal vasoconstriction and renal hemodynamic disturbances, increased levels of endothelin, impaired nitric oxide production, endothelial dysfunction, direct cellular toxicity due to relatively high tissue osmolality, and reperfusion injury via free radical formation and oxidative stress (OS).Citation4–7
Other than hydration, there is no generally accepted method of preventing CN. Although initial studies with N-acetylcysteine (NAC) found positive results, in subsequent studies, NAC’s efficiency could not be demonstrated.Citation8,Citation9
Carvedilol, a third-generation beta blocker with potent antioxidant and vasodilatory effects through its alpha blocker activity, theoretically possesses the potential to prevent CN. Carvedilol may prevent renal vasoconstriction and oxidative stress induced by CM. Nebivolol, another-third generation beta blocker, increases renal blood flow and glomerular filtration rate (GFR) by causing dilatation in the afferent and efferent arterioles. Furthermore, nebivolol has strong antioxidant properties caused by endothelial constitutive NO synthase (eNOS).Citation10
Oxidative stress and vasoconstriction appear to be important components of CN pathogenesis and, as mentioned above, both carvedilol and nebivolol are known to have vasodilatory and antioxidant effects. This study aimed to investigate whether carvedilol and nebivolol play preventive roles against development of CN and to compare the effects of each.
Materials and methods
Animals
This study was carried out in strict accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The experimental protocol was approved by the Animal Ethics Committee of the University of Adnan Menderes (2012/010). Forty male Wistar albino rats, aged 8 weeks and weighing 190–280 g, were enrolled into the study. During the study, the rats were housed at temperatures from 22–25 °C in 12-h day–night cycles. Except for during the dehydration period, rats had food and water within reach.
Experimental design and drugs
Rats were randomly divided into six groups, the control (C, n = 6), contrast material (CM, n = 6), carvedilol (CV, n = 7), carvedilol + contrast material (CV + CM, n = 7), nebivolol (N, n = 7), and nebivolol + contrast material (N + CM, n = 7) groups. To measure basal urea and creatinine values, blood samples were obtained through tail veins. Intravenous diatrizoate not exceeding 1% of the weights of the rats was administered by the tail vein following 3 days of dehydration, efficiency of which in inducing CN has been proved by Toprak et al. in their nebivolol study.Citation11 Carvedilol (Coronis®; Bilim, İstanbul, Turkey) and nebivolol (Nexivol®; Abdi İbrahim, İstanbul, Turkey) were obtained in pure forms and dissolved with 1% dimethyl sulfoxide (DMSO), constituting a solution such that 1 mL contained 1 mg carvedilol or nebivolol. At the initial phase of the study, to enhance the development of CN, all groups were deprived of water for 3 days. At that phase (between the first and third days), both carvedilol and nebivolol were given to the related groups (CV, CV + CM, N, and N + CM) at a dose of 2 mg/kg by way of oral gavage. Moreover, the control group and the CM group were given 0.9% NaCl fluid containing 1% DMSO at a dose of 2 mL/kg by way of orogastric gavage at that phase (between the first and third days). In the fourth day, following 3 days of dehydration (prevention from free access of water), sodium diatrizoate, which is a high osmolar ionic CM, was given to the CM, CV + CM, and N + CM groups under ether anesthesia and by way of the tail vein at a dose of 6 mL/kg. Then all groups were allowed to access free water until scarification. Final blood samples were obtained just before the scarification. Scarifications were performed 48 h after the administration of sodium diatrizoate. Drug administration, weight measurement, and blood sampling were performed between 09.00 and 10.00 h to be compatible with the circadian rhythm. For consistency, all blood was taken from the tail vein. Blood samples were stored at −80 °C until analyzed. In the 48th hour after administration of CM, the kidneys were removed under ether anesthesia. Right kidneys were kept in 10% formaldehyde solution to be used in pathological examination, and left kidneys were stored at −80 °C until tested for malondialdehyde (MDA), superoxide dismutase (SOD), and total antioxidant capacity (TAC).
Definition of contrast nephropathy
Contrast nephropathy is defined as either an increase of 0.5 mg/dL (44.2 μmol/L) or 25% in basal serum creatinine value 48 h after CM without any other reason or determination of significant histopathological changes in the renal tissue that can be attributable to contrast media.
Biochemical analysis
The rats’ serum urea and creatinine levels were measured using the Architect C8000 autoanalyzer and commercial kits from the Abbott Company. The urease methodology was used to test for urea, and the kinetic alkaline picrate method was used to test for creatinine.Citation12,Citation13 MDA measurement was performed using the Esterbauer method, which is a lipid peroxidation measurement method.Citation14
Dissected kidney tissues were immediately rinsed in ice-cold, phosphate-buffered saline. Tissues were homogenized (2000 rpm/min for 1 min, 1/10 w/v) using a stirrer (IKA Overhead Stirrer; IKA-Werke GmbH & Co. KG, Staufen, Germany) in 10% 150 mM phosphate buffer (pH 7.4) in an ice bath. The homogenate was centrifuged (Nüve-Bench Top Centrifuge NF 800R; Nüve, Ankara, Turkey) at 6000 × g for 10 min at 4 °C. Supernatants were frozen at −80 °C (Glacier Ultralow Temperature Freezer; Japan) until analyzed. Protein concentrations in supernatants were measured by a spectrophotometer (Shimadzu UV-1601; Shimadzu, Kyoto, Japan) using the Biuret method and commercially available kits (Archem Diagnostic Ind. Ltd., Istanbul, Turkey). Results are expressed as mg/mL protein.
TAC was determined using Real Assay Diagnostics kits (Product code: RL0017; Mega Tıp san., Gaziantep, Turkey). As they were analyzed in the tissue, TAC activity results were measured as Trolox equivalent/mg tissue protein.Citation15,Citation16
SOD enzyme values were determined using the Oxiselect Superoxide Dismutase Activity Assay commercial kit (Catalog Number: STA-340; Cell Biolabs İnc., San Diego, CA). The principle of this kit is based on the method modified by Sun et al. In this study, SOD activity was expressed as unit/mg (U/mg) tissue protein.Citation17
Histopathological evaluation
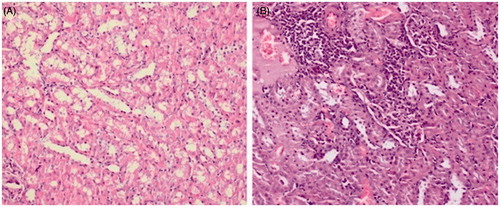
Tissue samples were fixed in 4% neutral buffered formalin for 24 h. Paraffin blocks had been prepared after routine tissue follow-up procedures, and 4-µm-thick sections were obtained from them and stained with hematoxylin–eosin. Then, samples were examined by light microscope for glomerulosclerosis, glomerular focal necrosis, expansion of the Bowman’s capsule, degeneration in the tubular epithelium, necrosis in the tubular epithelium, tubular dilatation, interstitial inflammation and congestion, thickening in the vascular wall, and interstitial fibrosis (). If all of the above were absent, the sample was graded as (−). If any were present, the sample was graded as mild (+), moderate (++), or severe (+++), depending on the degree of involvement.Citation18,Citation19
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) 19.0 program (SPSS Inc., Chicago, IL). Compatibility of quantitative data was tested using the Kolmogorov–Smirnov test. Parametric methods were used for variables that showed normal distribution, and non-parametric methods were used to analyze variables that did not show normal distribution. An independent t-test was used for paired comparison of independent groups, one-way ANOVA was used for comparison of multiple groups, the least significant difference (LSD) test was used on groups with an intergroup difference with homogeneous variance, and the Games–Howell test was used on groups with an inter-group difference without homogeneous variance. The repeated measures Anova test was used for repeated measurements of dependent, multiple groups. Chi-squared tests were used to compare categorical data. Differences were considered statistically significant if p < 0.05. All data were expressed as mean and standard error.
Results
Conditions of the rats at the baseline and in the follow-up
All rats tolerated carvedilol and nebivolol treatment and administration of diatrizoate well. There was no significant difference among groups in terms of urea and creatinine measurements (). After 3 days of dehydration, approximately 20% weight loss was observed in the rats. When dehydration was discontinued, water intake increased more than 2-fold and rat weights reached more than 95% of baseline weight.
Table 1. Multiple comparisons of urea and creatinine by experimental groups.
Renal functions
No group exhibited significant change in urea and creatinine values. In the CM group only, mean creatinine value increased from 0.505 to 0.585 mg/dL, although this change was not statistically significant (p = 0.067; ).
Oxidative stress markers
In the CM group, the renal tissue MDA level was significantly higher than in the C, CV, and NV groups (342.96 ± 11.58 vs. 260.13 ± 12.88, 270.94 ± 8.37, and 274.28 ± 4.68 nmol/mg protein, respectively, p < 0.001). Furthermore, the MDA level in the CM group was significantly higher than in the CVCM and NVCM groups (342.96 ± 11.58 vs. 302.82 ± 19.101 and 307.03 ± 16.47 nmol/mg protein, respectively, p < 0.001). There was no significant difference between MDA levels in the CVCM or NVCM groups as compared to the C group (p > 0.05).
The TAC level in the CM group was significantly lower than in all other groups (p < 0.001; ). There was no significant difference between TAC levels in the CVCM or NVCM groups as compared to the C group (p > 0.05). Although the SOD level in the CM group was slightly lower than in the other groups, this difference was not statistically significant ().
Table 2. Level of antioxidants and oxidant capacity.
Histopathological findings
In the CM group, degeneration in the tubule epithelium (p < 0.01), dilatation of the tubules (p < 0.05), and interstitial inflammation (p < 0.01) were significantly higher than in the other groups (). This means that interstitial inflammation, tubular degeneration, and tubular dilatation developed at lower rates in rats who received carvedilol and nebivolol before CM.
Table 3. Histopathological findings in renal tissue.
Furthermore, in all histopathological findings, there was no significant difference between the CVCM or NVCM groups and the C group (p > 0.05), supporting the existence of a protective role for both carvedilol and nebivolol against CM. This study found no significant difference among groups in vascular congestion, necrosis of the tubular epithelium, or expansion of the Bowman’s capsule (p > 0.05; ).
Discussion
Novel findings in this first study of the effects of nebivolol and carvedilol on CN are as follows: Both nebivolol and carvedilol decrease interstitial inflammation and tubular dilatation that occurs secondary to CM, and both decrease renal oxidative stress that occurs after CM administration. This study found no significant difference between the two agents in preventing CN.
Current recommendations to decrease the risk of CN include hydration with saline, avoiding high osmolar CM and a high volume of CM, and avoiding nephrotoxic drugs. N-acetylcysteine, ascorbic acid, and sodium bicarbonate treatments may be used, as they are fairly benign and inexpensive.Citation8,Citation9,Citation20,Citation21 One of the suggested mechanisms is renal artery vasoconstriction which leads to medullary hypoxia. Besides high osmolar CM, unbalanced oxidative stress, direct tubular toxicity, and tubular obstruction also play an important role in the pathogenesis of CN.Citation22
This study focused on oxidative stress, using high dose diatrizoate combined with volume depletion in an experimental CN model. Diatrizoate is known to be a high-osmolar-ionic CM which is known to be an important risk factor for developing CN.Citation23 Furthermore, dehydration has also been shown to potentiate the vasoconstrictive effects of CM which superimpose a prominent contribution on outer-medullary hypoxia.Citation24 In accordance with the literature, data from this study showed that administering diatrizoate combined with volume depletion significantly increased renal oxidative stress.
Free radicals may give harm to different cellular structures, such as phospholipids, proteins, and even DNA. Reactive oxygen species (ROS) have been shown to give direct harm to cells with a mechanism of protein oxidation/degradation and phospholipid peroxidation. The damage to cellular components, which leads dysfunction, has been demonstrated to be mainly related to phospholipid peroxidation and DNA.Citation25–28 Decreased antioxidant capability of the cell also plays a crucial role in the oxidative stress injury caused by increased ROS production.Citation26
Carvedilol is a non-selective beta-adrenoceptor agonist and alpha-adrenoceptor agonist. Moreover, carvedilol has remarkable antioxidant potential capable of scavenging free radicals and inhibiting ROS production.Citation29 Besides the parent compound, its metabolites such as: SB 211475, BM 910228, and SB 209995, are also powerful antioxidants and may provide significant contribution on carvedilol’s protective effect.Citation30 Previous studies have been demonstrated the antioxidant activity of carvedilol through in vitro test systems as well as animal models.Citation30–32 Yue et al. shown that carvedilol may inhibit superoxide anion release from activated neutrophils and lipid peroxidation in myocardial cell membranes.Citation30 In cultured endothelial cells, they demonstrated that carvedilol provides cellular protection against oxygen-radicals.Citation30 Treatment with carvedilol has been inferred to protect tissues from the damaging effects of ischemia-reperfusion injury (IRI) in rat limb and renal IRI models.Citation31–33 Two mechanisms have been proposed to explain the antioxidant activity of carvedilol: free iron chelation and/or free radical scavenging.Citation34,Citation35 Scavenging of free iron would lead to inhibition of hydroxyl radical formation from both hydrogen peroxide via Fenton reaction and superoxide anion via Haber–Weiss reaction.Citation36 This study demonstrated that pathological changes associated with CM injury were reduced in rats treated with carvedilol. The radical scavenging effects of carvedilol treatment in CN-induced rats were evident from increased levels of total antioxidant capacity and decreased levels of MDA resulting from oxidative stress. In this particular study, as compared with the control group we have found a tendency favoring carvedilol group to attenuate interstitial inflammation that occurred secondary to dehydration. Although, it did not reach statistical significance, the difference can be attributed to carvedilol’s anti-oxidant effects.
Nebivolol, a third-generation beta blocker that is a selective β1-adrenergic receptor antagonist, can be useful as a prophylactic agent for CN. It leads to an increase in renal NO excretion and a significant increase in renal plasma flow and GFR,Citation37 reduces endothelin-1,Citation38 and has an antioxidant effect,Citation39 all of which are related to the pathogenesis of CN. Based on these characteristics of nebivolol, Toprak et al. conducted an experimental study to investigate nebivolol’s protective effects,Citation11 finding that nebivolol decreased medullary congestion, proteinaceous casts, and tubular necrosis caused by CM. In addition, results showed that nebivolol had protective features against CN. In a previous study conducted with rats, treatment with nebivolol (2 mg/kg/day) induced a significant increase in renal NO excretion and, at a dose of 1 mg/kg, it significantly increased renal plasma flow and GFR.Citation40 This study gave nebivolol once daily at a dose of 1 mg/kg and demonstrated that both oxidative parameters and histopathological findings were in favor of the N + CM group as compared to the CM group. The dose of 2 mg/kg/day may provide better results; however, this study did not check the effects of higher doses of nebivolol.
This study’s CN model could not determine severe tubular necrosis, probably due to the induction method. The study did not use hard and invasive induction of CN, and this may explain why severe tubular necrosis was not seen in the CM group. Furthermore, the relatively low dose of diatrizoate administered might have contributed to the low level of histological findings. Although diatrizoate has been used at doses up to 10 mL/kg in rat studies, this study administered a dose of 6 mL/kg, in accordance with the design of Toprak et al.Citation11 This study showed that exposure to CM may not always be determined biochemically. Exposure to CM, especially at a low dose that does not cause a change in urea and creatinine levels, can lead to histopathological effects on renal tissue.
It has been shown that dehydration potentiates the vasoconstrictive effects of CM.Citation24 This study’s experimental design deprived rats of water for 48 h before exposure to CM. An interesting finding of the study was that the group that received carvedilol alone was protected from effects of dehydration as compared to the control group. Although not statistically significant, both interstitial inflammation and congestion were observed at lower rates in the group that received carvedilol alone compared to the control group. Carvedilol may provide additional protection to the kidneys, especially in patients with heart failure who encounter pre-renal problems due to excessive use of diuretics and in patients who enter coronary angiography in a dehydrated condition. However, this probable effect must be examined in larger studies.
The most common procedure associated with CN is coronary angiography, and most patients who underwent coronary angiography had indications for use of carvedilol and nebivolol. The demonstrated potential of both agents to protect the kidneys from CM harm may lead to new indications for them, which would provide protection from CN to this group of patients without any additional costs. Of interest recently Akgullu et al. compared the protective effects of metoprolol, nebivolol, and carvedilol against CN.Citation41 The incidents of CN were the lowest in the carvedilol group (4%) while the worst performance occurred in those taking metoprolol (10%). However, the difference between the groups in terms of the development of CN did not reach statistical significance. The number of patients in their study seems to be small enough to achieve certain results. Bigger clinical studies with beta blockers are needed in this clinical setting.
This study includes some limitations. Renal tissue was not examined by electron microscope, which might have provided further information about the mechanism in carvedilol and nebivolol that prevents CN. In addition, to determine kidney injury, it would have been better to use more sensitive markers, such as kidney injury molecule-1 or neutrophil gelatinase-associated lipocalin, to define acute kidney injury. In this particular study, we have demonstrated significant histopathological changes in renal tissue due to CM, however we did not observe significant changes in respect to creatinine values. This may have happened due to the design of experimental model. Before the rats were sacrificed, they were freely allowed to reach water for two days which may improve renal recovery.
The relatively low dose administered CM in this study might have contributed to the low level of histological findings and changes in oxidant–antioxidant parameters. Although diatrizoate has been used doses up to 10 mL/kg in rat studies, this study administered a dose of 6 mL/kg. Although it is known that a dose of 10 mL/kg increases the risk of CN, the design of this study predicted that a dose of 6 mL/kg would be appropriate, given that a dose of 10 mL/kg might lead to difficulty in demonstrating the efficiencies of the protective agents.
Conclusions
This study has confirmed that the protective effects of carvedilol and nebivolol likely are due to their radical-scavenging properties. Overall, these findings confirm that treatment with these agents can protect kidney tissue against oxidative stress resulting from CM. In addition, results provide rational support for use of carvedilol and nebivolol in clinical settings. The possibility of using nebivolol and carvedilol to protect from CN those patients for whom coronary angiography and coronary intervention are planned strongly encourages randomized human studies in this area.
Acknowledgments
The authors of this manuscript thank Biologist Ümit Afyoncu for his help with the laboratory work with the rats.
Declaration of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
References
- Goldfarb S, Mccullough P, Mcdermott J, Gay S. Contrast-induced acute kidney injury: Specialty-specific protocols for interventional radiology, diagnostic computed tomography radiology, and interventional cardiology. Mayo Clin Proc. 2009;84(2):170–179
- Mehran R, Nikolsky E. Contrast-induced nephropathy: Definition, epidemiology, and patients at risk. Kidney Int Suppl. 2006;100:11–15
- McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW. Acute renal failure after coronary intervention: Incidence, risk factors, and relationship to mortality. Am J Med. 1997;103(5):368–375
- Wong PC, Li Z, Guo J, Zhang A. Pathophysiology of contrast-induced nephropathy. Int J Cardiol. 2012;158(2):186–192
- Heyman SN, Rosenberger C, Rosen S. Regional alterations in renal hemodynamics and oxygenation: A role in contrast medium-induced nephropathy. Nephrol Dial Transplant. 2005;20:6–11
- Aydoğdu S. Contrast-induced nephropathy. Turk Kardiyol Dern Ars. 2013;41(1):28–30
- Romano G, Briguori C, Quintavalle C, et al. Contrast agents and renal cell apoptosis. Eur Heart J. 2008;29(20):2569, 7631–7634
- Yenicerioglu Y, Yilmaz O, Sarioglu S, et al. Effects of N-acetylcysteine on radio contrast nephropathy in rats. Scand J Urol Nephrol. 2006;40:63–69
- Marenzi G, Assanelli E, Marana I, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773–2782
- Oelze M, Daiber A, Brandes RP, et al. Nebivolol inhibits superoxide formation by NADPH oxidase and endothelial dysfunction in angiotensin II-treated rats. Hypertension. 2006;48:677–684
- Toprak O, Cirit M, Tanrisev M, et al. Preventive effect of nebivolol on contrast-induced nephropathy in rats. Nephrol Dial Transplant. 2008;23:853–859
- Talke H, Schubert GE. Enzymatic urea determination in the blood and serum in the Warburg optical test. Klin Wochenschr. 1965;43:174–175
- Fabiny DL, Ertingshausen G. Automated reaction-rate method for determination of serum creatinine with the Centrifi Chem. Clin Chem. 1971;17:696–700
- Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hidroxynonenal. Methods Enzymol. 1990;186:407–421
- Lowry O, Rosenbraugh N, Farr L, Rondall R. Protein measurement with the Folin-phenol reagent. J Biol Chem. 1951;193(1):265–275
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation more stabile ABTS radicalcation. Clin Biochem. 2004;37:277–285
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500
- Akgüllü Ç, Saruhan T, Eryilmaz U, et al. The first histopathological evidence of trimetazidine for the prevention of contrast-induced nephropathy. Ren Fail. 2014;İ36:575–580
- Boyacıoglu M, Turgut H, Akgullu C, Eryılmaz U, Kum C, Onbasılı OA. The effect of L-carnitine on oxidative stress responses of experimental contrast-induced nephropathy in rats. J Vet Med Sci. 2014;76:1–8
- Pannu N, Wiebe N, Tonelli M. Alberta kidney disease network, prophylaxis strategies for contrast-induced nephropathy. JAMA. 2006;295:2765–2779
- Spargias K, Alexopoulos E, Kyrzopoulos S, et al. Ascorbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation. 2004;110:2837–2842
- Detrenis S, Meschi M, Musini S, Savazzi G. Lights and shadows on the pathogenesis of contrast-induced nephropathy: state of the art. Nephrol Dial Transplant. 2005;20:1542–1550
- Toprak O, Cirit M. Risk factors for contrast-induced nephropathy. Kidney Blood Press Res. 2006;29:84–93
- Deray G, Baumelou B, Martinez F, Brillet G, Jacobs C. Renal vasoconstriction after low and high osmolar contrast agents in ischemic and nonischemic canine kidney. Clin Nephrol. 1991;36:93–96
- Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med. 2000;109:665–678
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460
- Paller MS. The cell biology of reperfusion injury in the kidney. J Investig Med. 1994;42:632–639
- Avunduk MC, Yurdakul T, Erdemli E, Yavuz A. Prevention of renal damage by alpha tocopherol in ischemia and reperfusion models of rats. Urol Res. 2003;31:280–285
- Dandona P, Ghanim H, Brooks DP. Antioxidant activity of carvedilol in cardiovascular disease. J Hypertens. 2007;25:731–741
- Yue TL, Mckenna PJ, Gu JL, Cheng HY, Ruffolo RR Jr, Feuerstein GZ. Carvedilol, a new antihypertensive agent, prevents lipid peroxidation and oxidative injury to endothelial cells. Hypertension. 1993;22:922–928
- Singh D, Chander V, Chopra K. Carvedilol attenuates ischemia–reperfusion induced oxidative renal injury in rats. Fundam Clin Pharmacol. 2004;18:627–634
- Akbas H, Ozden M, Kanko M, et al. Protective antioxidant effects of carvedilol in a rat model of ischemia-reperfusion injury. J Int Med Res. 2005;33:528–536
- Hayashi T, De Velasco MA, Saitou Y, et al. Carvedilol protects tubular epithelial cells from ischemia-reperfusion injury by inhibiting oxidative stress. Int J Urol. 2010;17:989–995
- Oettl K, Greilberger J, Zangger K, Haslinger E, Reibnegger G, Jürgens G. Radical-scavenging and iron-chelating properties of carvedilol, an antihypertensive drug with antioxidative activity. Biochem Pharmacol. 2001;62:241–248
- Noguchi N, Nishino K, Niki E. Antioxidant action of the antihypertensive drug, carvedilol, against lipid peroxidation. Biochem Pharmacol. 2000;59:1069–1076
- Baliga R, Ueda N, Walker PD, Shah SV. Oxidant mechanisms in toxic acute renal failure. Drug Metab Rev. 1999;31:971–997
- Feng MG, Prieto MC, Navar LG. Nebivolol-induced vasodilation of renal afferent arterioles involves β3-adrenergic receptor and nitric oxide synthase activation. Am J Physiol Renal Physiol. 2012;303(5):775–782
- Brehm BR, Wolf SC, Bertsch D, et al. Effects of nebivolol on proliferation and apoptosis of human coronary artery smooth muscle and endothelial cells. Cardiovasc Res. 2001;49:430–439
- Pires MJ, Rodríguez-Peña AB, Arévalo M, et al. Long-term nebivolol administration reduces renal fibrosis and prevents endothelial dysfunction in rats with hypertension induced by renal mass reduction. J Hypertens. 2007;25:2486–2496
- Greven J, Gabriëls G. Effect of nebivolol, a novel beta 1-selective adrenoceptor antagonist with vasodilating properties, on kidney function. Arzneimittelforschung. 2000;50:973–979
- Akgüllü C, Eryılmaz U, Güngör H, Huyut A, Zencir C, Hekim T. A clinical study about contrast nephropathy: Risk factors and the role of beta blockers. Anadolu Kardiyol Derg. 2014 Apr 28. doi: 10.5152/akd.2014.5304. [Epub ahead of print]