Abstract
Cyclosporine is used extensively in kidney transplantation and is a substrate for cytochrome P450 enzymes. The role of cytochrome p450 polymorphisms in kidney transplant outcome has not yet been fully elucidated. We investigate the clinical impact of single nucleotide polymorphisms in CYP3A4, CYP3A5, PPARα, and POR*28 in 255 kidney transplant recipients. We examine for any association with graft survival, time to first cancer, and delayed graft function, and also measure cyclosporine levels at days 3, 10, and months 1, 3, 6, and 12 after transplantation. The CYP3A4*22 allele is significant associated with the development of cancer post-kidney transplantation (HR 0.20, 95% CI 0.07–0.57, p = 0.003). It is not significantly associated with graft survival. No other SNP’s were associated with graft survival time to first cancer, or delayed graft function. There was a non-significant trend of lower cyclosporine dose requirement in CYP3A4*22 carriers. Independent replication of our findings is now warranted to confirm or reject the role of CYP3A variants in cancer development following kidney transplantation.
Introduction
The calcineurin inhibitor cyclosporine is an immunosuppressant drug used for the prevention of rejection following transplantation. Cyclosporine is a metabolic substrate for cytochrome P450 (CYP) 3A enzymes – in particular, CYP3A4 and CYP3A5 – and is transported out of cells via P-glycoprotein (ABCB1).Citation1 The introduction of cyclosporine transformed the care of transplant recipients, enabling a reduction in the incidence of acute rejection and an improvement in short-term graft survival.Citation2 However, the clinical use of cyclosporine has been limited by its narrow therapeutic index and its variable interindividual pharmacokinetics.Citation3 Therapeutic drug monitoring of cyclosporine is routinely performed, with the drug dosage adjusted according to whole-blood drug concentrations and patient clinical response.Citation3
An understanding of the influence of genetic factors on the pharmacokinetics of calcineurin inhibitors could allow identification of the optimal immunosuppressant drug combination, starting dose, and maintenance regimen for a particular individual, and help to identify patients with an increased risk of adverse effects. CYP3A4 is the most abundant isoform of cytochrome P450 (CYP) and it metabolizes approximately 45–60% of all prescribed drugs.Citation1 There is significant inter-individual variation in CYP3A4 activity. To date, more than 30 single nucleotide polymorphisms (SNPs) in the CYP3A4 gene have been identified.Citation4 A recently identified functional SNP located in CYP3A4 intron 6 (rs35599367 C > T) defines the CYP3A4*22 allele. The T-variant allele of CYP3A4*22 has been associated both with decreased hepatic CYP3A4 mRNA expression and with decreased CYP3A4 enzymatic activity.Citation5
Transplant recipients are at an increased risk of cancer post-transplantation.Citation6 A previous analysis of the National Renal Transplant Registry studied the incidence of skin cancer in Irish transplant patients and demonstrated a standardized incidence ratio for skin cancer in young transplant patients of as much as 250. There was also a statistically significant increase in the incidence rates for carcinoma in situ of the cervix, lung cancer, renal cancer, and lymphoma.Citation7 An association of melanocortin 1 receptor (MC1R) and methylenetetrahydrofolate reductase (MTHFR) polymorphisms with the development of post-transplant skin cancer has been demonstrated.Citation8
The aim of this study is to examine the association of CYP3A4 and CYP3A5 polymorphisms with graft survival and with the occurrence of cancer post kidney transplantation.
Methods
Ethics
The Beaumont Hospital Ethics Committee approved this study (protocol nos. 06/81 and RE: 07/16) and written informed consent was obtained from all participants. This study adhered to the Declaration of Istanbul.
Patients were adult, kidney only, deceased donor kidney transplant recipients transplanted in the period 1993–2002. All patients were white. Patients were maintained on standard triple immunosuppression with cyclosporine, prednisolone, and either mycophenolate mofetil or azathioprine. All DNA was collected at the time of transplantation.
Genotyping
DNA was extracted from whole blood using standard techniques. DNA samples for 292 patients were analyzed. Patients were part of a genome wide association study (GWAS), the results of which have been published elsewhere.Citation9 GWAS data were used to identify CYP3A5 allelic carrier status. In brief, genotyping was conducted using the Illumina Human 610-Quad platform (Illumina, Inc., San Diego, CA), which provides excellent coverage of common variation. The raw intensity data were processed and quality controlled using Illumina Genome Studio software (Illumina, Inc., San Diego, CA). Data were cleaned using PLINK employing standard protocols. CYP3A4*22 is not in linkage disequilibrium with any Hapmap SNPs and is, therefore, will not be detected in any GWAS studies. Allelic discrimination analysis was carried out for the determination of CYP3A4*22 allelic status (rs35599367). Allelic discrimination reaction was achieved using TaqMan (Applied Biosystems, Foster City, CA) genotyping assays (C__59013445_10) on the ABI PRISM 7500 Fast real-time PCR System (Applied Biosystems, Foster City, CA). There was sufficient phenotype data on 255 patients.
Statistical analysis
Patient demographic data were available from our renal patient database (Clinical Vision 3.4a Version 1.1.34.1, Clinical Computing, Cincinnati, OH). The log rank test for equality of survivor functions was used to compare graft survival outcomes between various groups. We also examined for an association with time to first cancer. Cancer data included all invasive cancers including non-melanoma skin cancer. Cancer data were obtained by merging the National Renal Transplant Database and the National Cancer Registry, Ireland, which registers all histologically confirmed cancers including non-melanoma skin cancer for the Republic of Ireland. Period at risk was from the date of transplant to death or end of follow up (July 2011).
Statistical analysis was performed using Stata (version 10, StataCorp, College Station, TX). For the univariate analysis of the association between categorical data (e.g., the incidence of acute rejection), we used Pearson’s Chi-squared test or Fisher’s exact test, as appropriate. Gene variants demonstrating evidence for an effect on Kaplan–Meier analysis (p < 0.05) were then analyzed in time-to-event analyses using a Cox model with the effect of genotype adjusted for relevant clinical variables. The dependent variables included gender, age of the recipient and donor, PRA, acute rejection, cold ischemia time, and delayed graft function. Due to the testing of multiple SNPs, the Bonferroni correction was applied and a p value of 0.0125 was considered statistically significant.
Results
Of the 255 patients studied, 230 (90.2%) had the CYP3A4*1/*1 genotype and 25 (9.8%) were heterozygous for the CYP3A4*22 (rs35599367) variant, whereas no variant homozygotes were found. The observed genotypes were in accordance with the Hardy–Weinberg equilibrium. describes the demographic characteristics of the study population. Patients carrying CYP3A4*22 variant were more likely to be older and have an older donor. No statistically significant difference was observed in graft survival between variant allele carriers and homozygotes for the reference allele ().
Figure 1. Kaplan–Meier test for graft survival based on the genotype for CYP3A4*1/*1 (CC) versus CYP3A4*22 (CT) (p = 0.57).
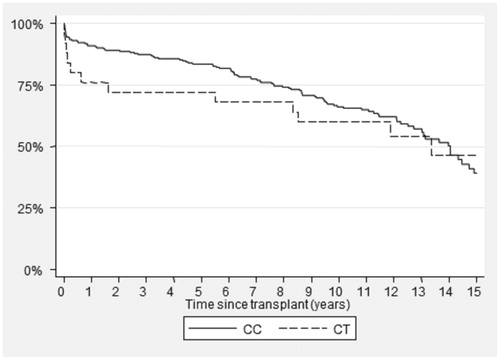
Table 1. Demographic characteristics of study population.
describes the cancer incidence in the study population. Eighty-seven patients developed cancer during the study period. The majority (84%) were non-melanomatous skin cancer. There were three patients who developed renal cell carcinoma, two cervical carcinomas, and one lymphoma. Other cancers included lung and colorectal carcinoma. demonstrates a lower incidence of cancer in patients carrying the CYP3A4*22 variant (cumulative incidence at 10 years, 16% vs. 36%, p = 0.03). This is despite the fact that patients with this variant were older than the CYP3A4*1/*1 group. We then performed a Cox proportional hazards model with time to first cancer as the dependent variable to correct for variables which may be significantly associated with time to first cancer (including acute rejection, gender, recipient age, degree of HLA mismatching, cold ischemic time). In this analysis, the association between CYP3A4*22 and time to first cancer was statistically significant (HR 0.20, 95% CI 0.07–0.57, p = 0.003) (). Recipient age and acute rejection were also significant.
Figure 2. Cumulative incidence of cancer – there is a lower rate of cancer in patients with CYP3A4*22 (CT) variant versus patients with CYP3A4*1/1 (CC) variant.
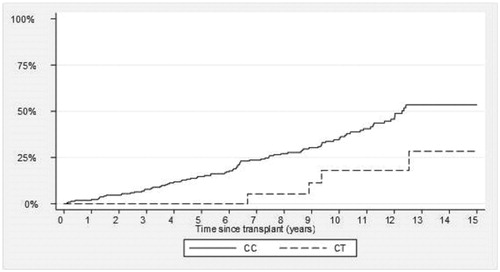
Table 2. CYP3A4 association with cancer.
Table 3. Cox regression model with time to first cancer as dependent variable.
There was no association between CYP3A4*22 and delayed graft function (data not shown).
CYP3A5
We genotyped the CYP3A5 allele and examined the association of gene variants with death-censored graft failure. There were three patients with CYP3A5*1/*1 genotype, 39 patients with CYP3A5*1/*3, and 213 patients with CYP3A5*3/*3. Given the low number of patients homozygous for the wild-type allele, the wild-type allele homozygotes and heterozygotes were grouped together for the purposes of the analysis. There was no significant association between CYP3A5 variants and graft survival, time to first cancer, and delayed graft function (data not shown).
SNPs within PPARα and POR*28
PPARα rs4253728 and rs4823613 and POR*28 rs1057868 were not associated with graft failure, DGF, or time to first cancer.
Cyclosporine levels
Cyclosporine trough levels were and doses were recorded at day 3, day 10, 1 month, 3 months, 6 months, and 12 months post-transplantation. When analyzing levels in the CYP3A4*1/*1 versus CYP3A4*22 groups, there was no significant difference between the groups when considering data at each time point of the follow-up. However, there was a consistent non-significant trend of a lower dose requirement (i.e., higher C0/dose) for CYP3A4*22 carriers. In the mixed model, CYP3A4*22 carriers show a +22.5% increased C0/dose compared with CYP3A4*1/*1 (p = 0.018).
Discussion
In our study, we have found a novel association of CYP3A4 variants with time to first cancer. In our patients, CYP3A4*22 variant is protective against cancer. The polymorphism is associated with lower hepatic expression of CYP3A4 enzyme and higher cyclosporine trough levels.Citation10,Citation11 The CYP3A4*22 allele is not in linkage disequilibrium (LD) with any HapMap SNPs, therefore, association studies using tag SNPs in genome wide association studies have failed to correlate this SNP with clinical phenotypes.Citation12
An association has previously been identified between the CYP3A4*22 variant and the occurrence of delayed graft function (DGF) following kidney transplantation.Citation12 The CYP3A4*22 allele was significantly associated with a higher risk of DGF compared with the CYP3A4*1/*1 patients after adjustment for known risk factors [odds ratio (OR) = 6.34, confidence interval (95% CI: 1.38–29.3), p = 0.015]. Previous studies have identified polymorphisms that have an association with cancer development in transplant patients. The presence of melanocortin 1 receptor (MC1R) and methylenetetrahydrofolate reductase (MTHFR) has previously been shown to have a strong association with the development of post-transplant skin cancer. A polymorphism of the MTHFR gene was identified at nucleotide position 677 on chromosome 1 with a C–T substitution which leads to amino acid change of alanine to valine and significantly reduced enzyme activity. This results in hypomethylation of genomic DNA and is strongly associated with the risk of SCC in renal transplant recipients.Citation8 A study of CYP3A4 polymorphisms in the general population identified an association with CYP3A4*1B polymorphisms with cancer risk, in particular for prostate cancer in African populations.Citation13
Malignancy was common in males in our patient group and this is similar to findings in other studies.Citation14 Cyclosporine is metabolized extensively in the liver and metabolism has been shown to generate reactive oxygen species (ROS) which have a role in cyclosporine associated nephrotoxicity.Citation15 We postulate that the increased generation of cyclosporine metabolites in patients with CYP3A4*1/*1, who have increased enzymatic activity in CYP3A, contributing to the increased incidence of cancer in this patient group identified in the study.
CYP3A5 is polymorphically expressed, with at least II SNPs identified to date. The most extensively studied CYP3A5 SNP involves an A–G transition at position 6986 within intron 3 of the CYP3A5 gene (rs776746). This SNP has recently been studied and did not show a relationship with graft survival in a large cohort of transplant patients.Citation16 We did not find an association between CYP3A5 and transplant outcomes. Carriers of the CYP3A5*1/*1 variant are expressers of CYP3A5 and have higher dose requirements and lower cyclosporine trough levels in comparison with CYP3A5*3/*3 carriers.Citation17,Citation18
A potential limitation of our study is that it is a single centre study of a small number of patients. Our study population was all white and, while this limits the possibility of population stratification, the results cannot be extrapolated to other populations.
All the patients in our study were treated with cyclosporine and a study of patients treated with tacrolimus is warranted. Cyclosporine and tacrolimus are both calcineurin inhibitors with similar mechanism of action and metabolism. Finally, we did not collect donor DNA. A study by Moore et al.Citation16 has demonstrated an association with donor CC genotype at C3435T within ABCB1 and kidney allograft failure.
In summary, this large study examines the association of recipient genotypes and important clinical endpoints in kidney transplantation. We found a statistically significant association between CYP3A4*22 and the development of cancer post-transplantation. Pre-transplant genotyping of this allele has the potential to identify patients at increased risk of adverse outcomes and could lead to enhanced surveillance and appropriate adjustment of immunosuppression in these patients. Our study now requires replication in a larger prospective cohort to confirm the role of CYP3A4 polymorphisms in renal transplant outcome.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Danielson PB. The cytochrome P450 superfamily: biochemistry, evolution and drug metabolism in humans. Curr Drug Metab 2002;3(6):561–597
- Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 2000;342(9):605–612
- Schiff J, Cole E, Cantarovich M. Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin J Am Soc Nephrol 2007;2(2):374–384
- Lamba JK, Lin YS, Schuetz EG, Thummel KE. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev 2002;54(10):1271–1294
- Wang D, Guo Y, Wrighton SA, Cooke GE, Sadee W. Intronic polymorphism in CYP3A4 affects hepatic expression and response to statin drugs. Pharmacogenomics J 2011;11(4):274–286
- Engels EA, Pfeiffer RM, Fraumeni JF, et al. Spectrum of cancer risk among US solid organ transplant recipients. J Am Med Assoc 2011;306(17):1891–1901
- Moloney FJ, Comber H, O'Lorcain P, O'Kelly P, Conlon PJ, Murphy GM. A population-based study of skin cancer incidence and prevalence in renal transplant recipients. Br J Dermatol 2006;154(3):498–504
- Laing ME, Dicker P, Moloney FJ, et al. Association of methylenetetrahydrofolate reductase polymorphism and the risk of squamous cell carcinoma in renal transplant patients. Transplantation 2007;84(1):113–116
- O'Brien RP, Phelan PJ, Conroy J, et al. A genome-wide association study of recipient genotype and medium-term kidney allograft function. Clin Transplant 2013;27(3):379–387
- Elens L, Bouamar R, Hesselink DA, et al. A new functional CYP3A4 intron 6 polymorphism significantly affects tacrolimus pharmacokinetics in kidney transplant recipients. Clin Chem 2011;57(11):1574–1583
- Elens L, van Schaik RH, Panin N, et al. Effect of a new functional CYP3A4 polymorphism on calcineurin inhibitors' dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenomics 2011;12(10):1383–1396
- Elens L, Bouamar R, Hesselink DA, Haufroid V, van Gelder T, van Schaik RH. The new CYP3A4 intron 6 C > T polymorphism (CYP3A4*22) is associated with an increased risk of delayed graft function and worse renal function in cyclosporine-treated kidney transplant patients. Pharmacogenet Genomics 2012;22(5):373–380
- Zhou LP, Yao F, Luan H, et al. CYP3A4*1B polymorphism and cancer risk: A HuGE review and meta-analysis. Tumour Biol 2013;34(2):649–660
- Farugia D, Mahoob S, Chesire J, et al. Malignancy related mortality following kidney transplantation is common. Kidney Int 2014;85:1395–1403
- de Arriba G, Calvino M, Benito S, Parra T. Cyclosporine A-induced apoptosis in renal tubular cells is related to oxidative damage and mitochondrial fission. Toxicol Lett 2013;218(1):30–38
- Moore J, McKnight AJ, Döhler B, et al. Donor ABCB1 variant associates with increased risk for kidney allograft failure. J Am Soc Nephrol 2012;23(11):1891–1899
- Gervasini G, Garcia M, Macias RM, Cubero JJ, Caravaca F, Benitez J. Impact of genetic polymorphisms on tacrolimus pharmacokinetics and the clinical outcome of renal transplantation. Transpl Int 2012;25(4):471–480
- Gijsen V, Mital S, van Schaik RH, et al. Age and CYP3A5 genotype affect tacrolimus dosing requirements after transplant in pediatric heart recipients. J Heart Lung Transplant 2011;30(12):1352–1359
