Abstract
Ophiocordyceps sinensis (O. sinensis; syn. Cordyceps sinensis) has been used in clinical therapy for diabetic kidney disease (DKD) for more than 15 years. O. sinensis is a household name in china and it is available even in supermarket. However, the precise role of O. sinensis has not been fully elucidated with meta-analysis. The aim of this study was to review existing evidence on the effectiveness of O. sinensis for the treatment of DKD. We identified 60 trials involving 4288 participants. Overall, O. sinensis combined with ACEI/ARB had a better effect when compared to ACEI/ARB alone on 24 h UP (MD = −0.23 g/d, 95% CI: − 0.28 to −0.19, p < 0.00001), UAER (MD = −19.71 μg/min, 95% CI: −22.76 to −16.66, p < 0.00001), MAU (MD = −45.09 mg/d, 95% CI: −55.68 to −34.50, p < 0.00001), BUN (MD = −0.70 mmol/L, 95% CI: −1.02 to −0.39, p < 0.0001), SCr (MD = −8.37 μmol/L, 95% CI: −12.41 to −4.32, p < 0.0001), CRP (MD = −1.32 mg/L; 95% CI: −1.78 to −0.86; p < 0.00001), TG (MD = −0.51 mmol/L; 95% CI: −0.69 to −0.34, p < 0.00001), TC (MD = −0.64 mmol/L; 95% CI: −0.91 to −0.37, p < 0.00001), and SBP (MD = −2.01 mmHg; 95% CI: −3.45 to −0.58, p = 0.006). However, no effects were found for DBP, FBG, and HbA1C. This meta-analysis suggested that use of O. sinensis combined with ACEI/ARB may have a more beneficial effect on the proteinuria, inflammatory, dyslipidemia status as compared to ACEI/ARB alone in DKD III–IV stage patients, while there is no evidence that O. sinensis could improve the hyperglycemia status. However, with regard to low-quality and significant heterogeneity of included trials, to further verify the current results from this meta-analysis, long-term and well-designed RCTs with high-quality study are warranted to ascertain the long-term efficacy of O. sinensis.
Introduction
Diabetic kidney disease (DKD), a common microvascular complication of diabetes, is a leading cause of end-stage renal disease (ESRD) worldwide.Citation1 The successive manifestations of DKD were high glomerular filtration rate (GFR), microalbuminuria, albuminuria, and ESRD. If left untreated, the resulting uremia is fatal. DKD is reversible in both microalbuminuria and albuminuria. However, once the disease reaches azotemia, the progression of DKD becomes irreversible.Citation2 Therefore, it is imperative to find early and effective ways to treat this disease.
The inhibition of the renin–angiotensin system (RAS), including angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB), has been shown to reduce albuminuria and delay the progression of DKD, and has become the standard treatment for albuminuric patients.Citation3,Citation4 However, in spite of the renoprotective effects of ACEI and ARB, DKD still progresses to ESRD in a large proportion of patients.Citation5 This indicates that, in addition to RAS, other pathways are involved in the pathogenesis of ESRD. Several recent studies have demonstrated that traditional Chinese medicine (TCM) combined with ACEI/ARB treatment can safely and effectively treat DKD.Citation6,Citation7
The caterpillar fungus Ophiocordyceps sinensis (syn. Cordyceps sinensis), one of the most famous TCMs and an ingredient in Chinese herbs is an enteropathogenic fungus that belongs to the family Clavicipitaceae, division Ascomycotina. O. sinensis is also called “Dong Chong Xia Cao” or “winter worm summer grass”. Several active components have been extracted and purified from O. sinensis, such as cordycepin and its derivatives, polysaccharides, trace elements, mycelium, and melanin.Citation8,Citation9 These components have various physiological properties, such as anti-oxidative, anti-fibrogenic, anti-tumor, anti-thrombotic, anti-viral, anti-fungal, and anti-inflammatory activities.Citation10–13 In recent decades, artificial cultivated fungus and curative products derived from the so-called “Cordyceps” in various forms such as capsules (e.g. jin shui bao capsule, bai ling capsule, zhi ling capsule) has been applied in clinical therapy for DKD. However, its efficacy has not been fully identified. In this meta-analysis, we aimed to systematically review the evidence regarding the benefits of O. sinensis in the treatment of DKD.
Methods
Data sources and search strategies
A systematic review of the published literature was performed according to the approach recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for conducting meta-analyses.Citation14 MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CCRCT), the Chinese Biomedical Literature (CBM), China National Knowledge Infrastructure (CNKI), Wang Fang, and VIP databases were searched to identify eligible studies (all to September 2014). The following search terms were used: Ophiocordyceps sinensis, Cordyceps sinensis, O. sinensis, C. sinensis, cordycepin, Cordyceps militaris, winter worm summer herb, dong chong xia cao, bai ling capsule, jin shui bao capsule, ACEI or angiotensin-converting enzyme inhibitors or ACE inhibitors, ARB or angiotensin receptor blockers, DKD or diabetic kidney disease, diabetic nephropathy, and kidney disease ( shows details of the search method used in this meta-analysis). The search was limited to randomized controlled trials (RCTs) comparing O. sinensis combined with ACEI/ARB with ACEI/ARB alone without language limitation.
Inclusion criteria
Types of studies
Published reports of randomized controlled trials (RCTs) comparing O. sinensis + ACEI/ARB and with ACEI/ARB alone in patients diagnosed with DKD stage III or IVCitation15,Citation16 with available data for our prescribed outcomes, while without language limitation.
Type of participants
The studies were restricted to any patients who were diagnosed with DKD stage III or IV according to Mogensen staging.Citation16
Type of interventions
Studies comparing O. sinensis + ACEI/ARB and with ACEI/ARB alone for DKD patients. The form of O. sinensis was artificial cultivated fungus, while these studies with the form of O.sinensis were a decoction or formula were excluded.
Type of outcome measures
Proteinuria parameters: 24-h proteinuria (24hUP), urinary albumin excretion rate (UAER), microalbuminuria (MAU); Renal Function parameters: serum creatinine (SCr), blood urea nitrogen (BUN); Inflammatory parameters: C-reactive protein (CRP); Lipid parameters: serum triglycerides (TG), total cholesterol (TC) levels; Blood pressure parameters: systolic blood pressure (SBP), diastolic blood pressure (DBP); Blood glucose parameters: fasting blood glucose (FBG), and glycated hemoglobin A1C (HbA1c).
Data extraction and management and assessment of study quality
Data on participants were extracted from all referenced studies to retrieve the characteristics of the study sample, baseline of the study, and intervention characteristics for each group. Two reviewers (Y. L. and S. K. Y.) extracted data independently. Disagreements were resolved after discussion with a third reviewer (L. X.). Outcomes included the level of 24hUP, UAER, and MAU; BUN and SCr; CRP; TC and TG; SBP and DBP; and FBG and HbA1c. The validated Jadad scale was used to evaluate the quality of data from each study.Citation17 The Jadad score included the following components: randomization (0–2 points), double-blinding (0–2 points), and description of withdrawals and dropouts (0–1 point). Allocation concealment was estimated by the criteria adopted from Schulz et al.Citation18 Studies with Jadad scores of ≥3 were regarded as being of high quality.
Data analysis
Meta-analysis was performed using Review Manager version 5.2 (The Cochrane Collaboration, Oxford, UK). The mean change in each study from baseline to end point was considered a continuous variable. Continuous data using the same unit were presented as the mean difference (MD) with 95% CI. The chi-squared test for heterogeneity (defined as significant when I2 is >50% or p < 0.1) was performed. The random effect model was used for the meta-analysis when there was significant heterogeneity. If there was not significant heterogeneity, the fixed effect model was used for the analysis.
Results
Search results
Of the 794 studies identified through online searches, 236 were ruled out because duplicates were found. The full text of the remaining 558 studies was retrieved, of which 385 were removed on review of the titles and abstracts. Among these, non-RCTs, reviews, comments, and animal studies were excluded. After further reading, 85 studies did not compare O. sinensis + ACEI/ARB with ACEI/ARB alone, 11 studies lacked clear information on dosage and outcomes of treatments, 9 studies contained unauthenticated data, 4 studies contained no outcomes of interest, the form of O. sinensis is decoction or formula in three studies, and the follow up was too short in one studies. At the end of the review process, 60 studies were included in the meta-analysis, 42 studies compared O. sinensis + ARB with ARB alone, and 18 compared O. sinensis + ACEI with ACEI alone. shows a chart of the study selection process.
Study characteristics and quality assessment
The main characteristics of the selected studies are summarized in . The 60 articles comprising 4288 participants (2163 in the experimental group and 2125 in the control group) were published in Chinese between 2005 and 2013. The median sample size was 72 (range, 36–205). Three kinds of O. sinensis were analyzed in this study: 25 articles discussed jin shui bao capsule (JSB), 32 discussed bai ling capsule (BL), and 3 discussed zhi ling capsule (ZL). The Jadad score of included study were listed in and most trials were of low quality. Only 7 of 60 studies with Jadad score = 3 were regarded as being of high quality.Citation19–25 Five articles were described as having used random number tables.Citation19–23 None of the articles reviewed contained clear methods used for allocation concealment. Blinding was not confirmed. However, the outcomes were not likely to be influenced from any lack of blinding. In all studies, the characteristics of the participants in the different groups were similar at baseline (age, sex, disease course, etc.).
Table 1. Characteristics of included studies.
Efficacy assessment
Effects on proteinuria (24hUP, UAER, and MAU)
As shown in , the effects on proteinuria had three indicators containing 24hUP, UAER, and MAU. Data regarding the effects of therapy using O. sinensis + ACEI/ARB compared to that using ACEI/ARB alone on 24hUP were available from 23Citation1Citation9,Citation20,Citation23,Citation26–45 of the 60 trials comprising 1714 participants. Based on the results of the meta-analysis, therapy with O. sinensis + ACEI/ARB decreased 24hUP by −0.23 g/d (95% CI: −0.28 to −0.19) compared to that in the control (p < 0.00001; and ). There was evidence of significant heterogeneity in the magnitude of effects across these trials (I2 = 88%, p for heterogeneity <0.00001, , ); therefore, the random-effects model was used. Overall, O. sinensis + ACEI/ARB decreased 24hUP according to the present meta-analysis.
Figure 2. (A) Forest plot of studies comparing the effects of O. sinensis + ACEI/ARB with ACEI/ARB alone on 24hUP (g/d) in DKD. (B) Forest plot of studies comparing the effects of O. sinensis + ACEI/ARB with ACEI/ARB alone on UAER (μg/min) in DKD. (C) Forest plot of studies comparing the effects of O. sinensis + ACEI/ARB with ACEI/ARB alone on MAU (mg/d) in DKD.
Table 2. Summary effect of O. sinensis + ACEI/ARB on DKD patients.
Data for the effects of therapy using O. sinensis + ACEI/ARB compared to using ACEI/ARB alone on UAER were available from 25 trials comprising 1737 participants.Citation20,Citation21,Citation24,Citation27,Citation31,Citation38,Citation46–64 In general, therapy with O. sinensis + ACEI/ARB decreased UAER by 19.71 µg/min (95% CI: −22.76 to −16.66) compared with that of ACEI/ARB alone (p < 0.00001; and ). There was evidence of significant heterogeneity in the magnitude of effects across these trials (I2 = 79%, p for heterogeneity < 0.00001; and ).
Eighteen trialsCitation22,Citation24,Citation25,Citation47,Citation50,Citation55,Citation65–76 comprising 1347 participants had outcomes for MAU. The meta-analysis showed that therapy with O. sinensis + ACEI/ARB decreased MAU by 45.09 mg/d (95% CI: −55.68 to −34.50) compared with that of the control (p < 0.00001; and ). There was evidence of significant heterogeneity in the magnitude of effects across these trials (I2 = 99%, p for heterogeneity <0.00001; and ). In all, therapy using O. sinensis + ACEI/ARB was more effective in reducing MAU as well as 24hUP and UAER. Meanwhile, a subgroup analysis was performed that was stratified by the study duration. The results also showed that there was a significant difference on 24hUP and MAU among trials of different duration.
Effects on renal function (BUN, SCr)
As seen in , the effect on renal function has two parameters: BUN and SCr. Twenty-seven trials comprising 2062 participantsCitation19,Citation24–26,Citation30–32,Citation34–37,Citation39,Citation41,Citation43–46,Citation50,Citation52,Citation58,Citation60,Citation62,Citation69,Citation70,Citation72,Citation76,Citation77 reported effects on BUN. In summary, therapy with O. sinensis + ACEI/ARB decreased BUN by 0.70 mmol/L (95% CI: −1.02 to −0.39) compared with that of ACEI/ARB alone (p < 0.0001; and ). There was evidence of significant heterogeneity in the magnitude of effects across these trials (I2 = 89%, p for heterogeneity <0.00001; and ).
Figure 3. (A) Forest plot of studies comparing the effects of O. sinensis + ACEI/ARB with ACEI/ARB alone on BUN (mmol/L) in DKD. (B) Forest plot of studies comparing the effects of O. sinensis + ACEI/ARB with ACEI/ARB alone on SCr (μmol/L) in DKD.
Forty-four trials comprising 3168 participantsCitation19,Citation20,Citation23–26,Citation28,Citation30–32,Citation34–39,Citation41–46,Citation48–53,Citation56,Citation57,Citation60,Citation62,Citation64,Citation65,Citation68–72,Citation74–78 showed results for SCr levels. Overall, therapy with O. sinensis + ACEI/ARB decreased SCr by 8.37 µmol/L (95% CI: −12.41 to −4.32) compared to that of ACEI/ARB alone (p < 0.0001; and ). There was evidence of significant heterogeneity in the magnitude of effects across these trials (I2 = 95%, p for heterogeneity <0.00001; and ). It is indicated that therapy with O. sinensis + ACEI/ARB has an advantage over therapy with ACEI/ARB alone in reducing BUN and SCr. In addition, a subgroup analysis was conducted according to study duration. The results on BUN and SCr among trials of different duration were in accordance with the results of whole studies.
Effects on inflammatory biomarkers (CRP)
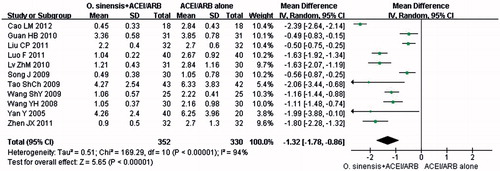
The changes in CRP were analyzed from 11 trials comprising 682 participants.Citation20,Citation45,Citation46,Citation49,Citation51,Citation52,Citation56–58,Citation62,Citation78 There were significant changes in CRP (p < 0.00001, MD: −1.32 mg/L; 95% CI: −1.78 to −0.86; and ). As there was evidence of heterogeneity (p < 0.00001, I2 = 94%; and ), the random-effects model was used for meta-analysis.
Effects on blood lipids (TG, TC)
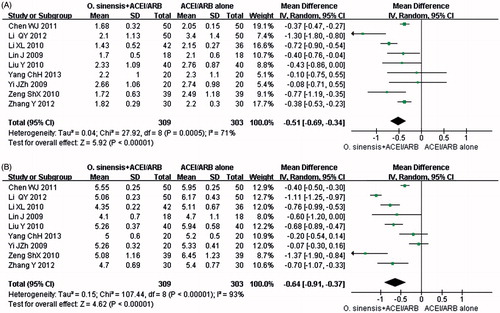
The meta-analysis on blood lipids had two parameters: TG and TC. TG and TC were compared among patients receiving O. sinensis + ACEI/ARB and those receiving ACEI/ARB alone in nine trials comprising 612 patients.Citation22,Citation24,Citation30,Citation39,Citation42,Citation43,Citation61,Citation69,Citation72 The significant difference was identified in terms of the TG (p < 0.00001, MD: −0.51 mmol/L, 95% CI: −0.69 to −0.34; and ) and TC (p < 0.00001, MD: −0.64 mmol/L, 95% CI: −0.91 to −0.37; and ); therefore, it can be concluded that therapy with O. sinensis + ACEI/ARB was more effective in ameliorating blood lipids than therapy with ACEI/ARB alone.
Effects on blood pressure (SBP, DBP)
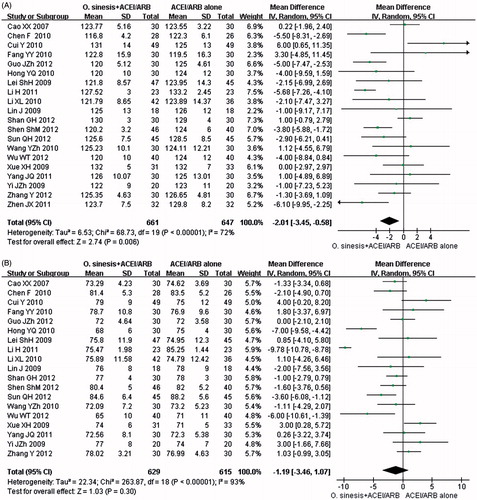
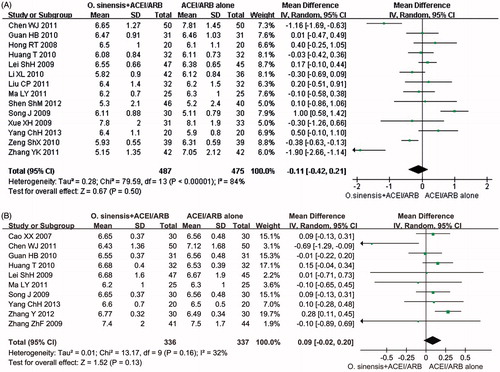
As shown in , the effects on SBP and DBP were assessed in 20 trials comprising 1308 participantsCitation24,Citation26,Citation28,Citation30,Citation34,Citation35,Citation37,Citation39,Citation41,Citation44,Citation47,Citation55,Citation61–64,Citation68,Citation73,Citation76,Citation77 and 19 trials comprising 1244 participants,Citation24,Citation26,Citation28,Citation30,Citation34,Citation35,Citation37,Citation39,Citation41,Citation44,Citation47,Citation55,Citation61,Citation63,Citation64,Citation68,Citation73,Citation76,Citation77 respectively. Based on the results of the meta-analysis, the groups that received therapy with O. sinensis + ACEI/ARB showed a significant decrease in SBP compared to that of the controls (P = 0.006, MD: −2.01 mmHg, 95% CI: −3.45 to −0.58; and ); however, a non-significant decrease was found for DBP (p = 0.30, MD: −1.19 mmHg, 95% CI: −3.46 to 1.07; and ).
Effects on blood glucose (FBG, HbA1c)
Fourteen trials comprising 962 participantsCitation20,Citation22–24,Citation27,Citation48,Citation49,Citation53,Citation55,Citation64,Citation68,Citation69,Citation72,Citation78 and 10 trials comprising 673 participantsCitation20,Citation27,Citation53,Citation61,Citation63,Citation64,Citation69,Citation70,Citation72,Citation78 reported the effects on FBG and HbA1c, respectively. The meta-analysis indicated that therapy using O. sinensis + ACEI/ARB produced no statistically significant change in FBG compared to that of therapy using ACEI/ARB alone (p = 0.50, MD: −0.11 mmol/L, 95% CI: −0.42 to 0.21; and ). In addition, the combined therapy did not decrease the level of HbA1C (p = 0.13, MD: 0.09%, 95% CI: −0.02 to 0.20; and ).
Investigations of heterogeneity
We performed subgroup analysis to exploring mean change in proteinuria, renal function, inflammatory, blood lipid, blood pressure, and blood glucose parameters; the analysis was stratified by different kinds of artificial cultivated fungus capsules, different study duration, and different character of the controlled group. As shown in , the results of subgroup analysis showed that there was no difference on 24hUP, UAER, MAU, TC, DBP, and FBG levels among trials of different kinds of artificial cultivated fungus capsules or different study duration. In addition, use of O. sinensis could both effectively improve these parameters when compared with ACEI or ARB. However, therapy with zhi ling capsule(ZL) has no effect on serum BUN and Scr. Encouragingly, Funnel plots for some key outcomes such as 24hUP, UAER, MAU, BUN, SCr, CRP, TG, TC, SBP, DBP, FBG, and HbA1C were asymmetric ( and ), which suggested there was publication bias among these studies.
Discussion
This systematic review and meta-analysis have consolidated data from a great deal of RCTs administering therapy with O. sinensis + ACEI/ARB to patients with DKD. In summary, O. sinensis + ACEI/ARB appears to have significantly reduced proteinuria (24hUP, UAER, MAU) and improved renal function (BUN, SCr) compared with therapy using ACEI/ARB alone. Furthermore, O. sinensis combined + ACEI/ARB decreased the level of CRP, TG, TC, and SBP; however, a non-significant decrease was found for DBP, FBG, and HbA1c. If confirmed in larger high-quality studies, these results suggest that therapy using O. sinensis + ACEI/ARB might play a role in delaying or even preventing the progression of DKD.
DKD is the most devastating and costly complication in patients with diabetes worldwide. The pathogenesis of DKD is complicated and not yet fully understood. In addition to RAS, other pathways, such as oxidative stress,Citation79 polyol,Citation80 protein kinase C (PKC) activation,Citation81 inflammation,Citation82 and excessive production of advanced glycation end products (AGEPs)Citation83 also contribute to the occurrence and development of DKD, especially oxidative stress and the production of reactive oxygen species(ROS).Citation84,Citation85 It is known that treatment with ACEI/ARB can slow the progression of DKD to ESRD; however, it does not stop or reverse the process.
Ophiocordyceps sinensis (syn. C. sinensis) (Berk.) Sacc, a fungus highly valued in China as a tonic and herbal medicine, was discovered 2000 years agoCitation86 and its use was documented formally in the Qing Dynasty and mentioned in the Bencao Congxin (New Compilation of Materia Medica) in 1757. Ophiocordyceps sinensis parasitizes the larvae of moths (Lepidoptera), particularly Hepialus armoricanus, and uses the nutrients from each larva to create mycelia and replace the larval body with sclerotium, from which the stroma and fruiting body grow. Several kinds of active components have been extracted and purified from O. sinensis, such as cordycepin and its derivatives, polysaccharides, trace elements, mycelium, and melanin.Citation8,Citation9 Ophiocordyceps sinensis is now used for multiple medicinal purposes, such as anti-inflammatory, antitumor activity, immunostimulating properties, anti-microbial, anti-fibrotic, and neuroprotective effects,Citation87,Citation88 due to its various physiological properties. Several experiments have proved that the chemical components, pharmacology, and toxicology of natural and cultured O. sinensis are similar; therefore, natural O. sinensis can be replaced by cultured O. sinensis.Citation89
Oxidative stress and increased production of ROS plays an important role in the occurrence and development of DKD. Our previous studyCitation90 demonstrated that cordycepin (3′-deoxyadenosine) could ameliorate the albumin-induced epithelial–mesenchymal transition of tubular cells (HK2) by decreasing Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase activity and inhibiting ROS production. The trace elements in C. sinensis, which serve as coenzymes of glutathione peroxidase, can reduce mesangial cell contractility and oxidative stress.Citation91 In vitro and in vivo studies have proved that a polysaccharide isolated from O. sinensis and containing glucose, mannose, and galactose exhibited strong antioxidant activity, acted as a direct free-radical scavenger, and might have attenuate renal failure.Citation92,Citation93 The aqueous extract of the Indian species of Ophiocordyceps sinensis has spectacular antioxidant and anti-inflammatory capabilities.Citation94 In addition, the melanin derived from O. sinensis also exhibited chelating ability and antioxidant activity.Citation95 Superoxide dismutase (SOD) can eliminate superoxide anion and inhibit lipid peroxidation. C. sinensis can elevate SOD and decrease the biomarkers of oxidative stress (such as methylenedioxyamphetamine and advanced oxidation protein products).Citation96
Aldose reductase (AR), the first and rate-limiting enzyme of the polyol pathway that converts glucose to fructose, plays a key role in the pathogenesis of DKD.Citation97 Previous studies have shown that bailing capsule can inhibit AR.Citation91 Therefore, we speculate that the nephroprotection of O. sinensis is partially a result of that. In addition, several studies have demonstrated that the extracts from C. sinensis could reduce the pressure within glomeruli and decrease the capillary hydrostatic pressure, thus lowering glomerular hyperfiltration and preventing the development of glomerulosclerosis.Citation98
Previous study has demonstrated that nephrin loss is a main cause of proteinuria.Citation99 Evidence from animal studies showed that DKD was ameliorated with bailing capsule, which may be related to the restoration and gene expression of nephrin.Citation100 Transforming growth factor-β1 (TGF-β1) and connective tissue growth factor (CTGF) are indispensable during the pathogenesis of DKD.Citation101 Cordycepin (3′-deoxyadenosine) interferes with the TGF-β1 and bone morphogenetic protein signaling through downregulation of Smads, which might be the underlying anti-fibrotic effect of this agent.Citation102 Moreover, cordycepin markedly blocks TGF-β1-mediated activation of renal interstitial fibroblast cells through up regulating anti-fibrotic hepatocyte growth factor (HGF) at both the gene and protein levels.Citation103 In addition, one study proved that bailing capsule can reduce the expression of TGF-β1 and CTGF in rats with DKD, which can reduce renal tissue injury.Citation104
Hyperlipidemia, a risk factor for DKD, can stimulate cell proliferation and induce glomerulosclerosis. Recent studies showed that cordycepin prevents hyperlipidemia by activating AMP-activated protein kinase, which plays a critical role in the regulation of fat metabolism.Citation11,Citation105 A polysaccharide (O. sinensis-F30) obtained from the cultured mycelium of O. sinensis lowered the plasma TG and TC levels in genetic diabetic mice after intraperitoneal administration.Citation106 In addition, C. sinensis has anti-lipid peroxidation properties and inhibits accumulation of cholesteryl, a cholesterol ester, in macrophages by suppressing low-density lipoprotein oxidation.Citation107
The increases of both hypoxia inducible factor-1 (HIF-1α) and vascular endothelial growth factor (VEGF) in the kidneys of rats with DKD, and the positive correlation suggests that there is chronic hypoxia in the renal tissue of those with DKD. C. sinensis might protect against chronic hypoxia injury in those with DKD by lowering the expression of HIF-1α and VEGF.Citation108 Caspase-3 is a key enzyme of apoptosis that is closely related to kidney injuries. The protective effect of C. sinensis may be inhibiting the activation of caspase-3 during the AngII-induced apoptosis of NRK-52 E.Citation109 Moreover, bailing capsule can protect renal tubular Na+–K+ATP enzyme, stimulate the production of epidermal growth factor from renal tubular epithelial cells, improve immunologic competence, and motivate renal tubular epithelial cell proliferation.Citation110 In summary, O. sinensis might protect renal tissues through antioxidant activity, inhibiting AR, ameliorating glomerular hyperfiltration, elevating nephrin levels, restraining renal interstitial fibrosis, and reducing hyperlipidemia. The underlying mechanism and potential effects of O. sinensis on DKD warrant further investigation.
There are several important potential study limitations to this systematic review. The major limitation was the poor quality of the trials. Most of the trials were of very low methodological quality and the interpretation of any positive findings for the efficacy of the included O. sinensis for treating DKD should be made with caution. Second, all trials were published in Chinese, creating the possibility of publication bias. Third, adverse effects were not described in detail. Only 14 RCTs reported any adverse events, and none were serious. Fourth, most of the included studies reported short-term (<1 year) outcomes of O. sinensis supplementation treatment, the long-term efficacy of O. sinensis need to be proven by further long-term studies. Finally, there was evidence of heterogeneities of baseline clinical features in these included RCTs, such as different study duration and different types of artificial cultivated O. sinensis. We tried to control some of these differences by performing subgroup analysis according to the level of baseline characters and using random-effect models in our analysis, nevertheless, we must have to take note that the accuracy of the pooled analytical results might be influenced.
Conclusion
Taken together, use of O. sinensis combined with ACEI/ARB may have a more beneficial effect on the proteinuria, inflammatory, dyslipidemia status as compared to ACEI/ARB alone in DKD III–IV stage patients, while there is no evidence that O. sinensis could improve the hyperglycemia status. However, with regard to low-quality and significant heterogeneity of included trials, therefore, the clinical application should be concerned with more long-term high-quality and well-designed RCTs to ascertain the clinical value of O. sinensis as an additional therapeutic option for DKD patients.
Acknowledgements
Y. L., S. K. Y., X. Z., M. W., and D. T. generated the data for the manuscript and wrote the manuscript. F. Y. L. and L. S. edited the manuscript. X. L. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of interest
The authors declare that there is no conflict of interests regarding the publication of this paper. This study was supported by grants from the National Natural Science Foundation of China (81370832 and 81100541).
References
- Packham DK, Alves TP, Dwyer JP, et al. Relative incidence of ESRD versus cardiovascular mortality in proteinuric type 2 diabetes and nephropathy: Results from the DIAMETRIC (Diabetes Mellitus Treatment for Renal Insufficiency Consortium) database. Am J Kidney Dis. 2012;59(1):75–83
- Osta V, Natoli V, Dieguez S. Evaluation of two rapid tests for the determination of microalbuminuria and the urinary albumin/creatinine ratio. An Pediatr (Barc). 2003;59(2):131–137
- Ting RZ, Luk AO, Chan JC. Treatment and landmark clinical trials for renoprotection. Contrib Nephrol. 2011;170:184–195
- KDOQI. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49(2 Suppl 2):S12–S154
- Luk A, Chan JC. Diabetic nephropathy – What are the unmet needs? Diabetes Res Clin Pract. 2008;82 (Suppl 1):S15–S20
- Xiao Y, Liu Y, Yu K, et al. The effect of chinese herbal medicine on albuminuria levels in patients with diabetic nephropathy: A systematic review and meta-analysis. Evid Based Complement Alternat Med. 2013;2013:937549
- Liu X, Liu L, Chen P, et al. Clinical trials of traditional Chinese medicine in the treatment of diabetic nephropathy – a systematic review based on a subgroup analysis. J Ethnopharmacol. 2014;151(2):810–819
- Lo HC, Hsieh C, Lin FY, Hsu TH. A systematic review of the mysterious caterpillar fungus in Dong-ChongXiaCao (Dong Chong Xia Cao) and related bioactive ingredients. J Tradit Complement Med. 2013;3(1):16–32
- Yue K, Ye M, Zhou Z, Sun W, Lin X. The genus Cordyceps: A chemical and pharmacological review. J Pharm Pharmacol. 2013;65(4):474–493
- Wang Y, Yin H, Lv X, et al. Protection of chronic renal failure by a polysaccharide from Cordyceps sinensis. Fitoterapia. 2010;81(5):397–402
- Guo P, Kai Q, Gao J, et al. Cordycepin prevents hyperlipidemia in hamsters fed a high-fat diet via activation of AMP-activated protein kinase. J Pharmacol Sci. 2010;113:395–403
- Cho HJ, Cho JY, Rhee MH, et al. Inhibitory effects of cordycepin (3'-deoxyadenosine), a component of Cordyceps militaris, on human platelet aggregation induced by thapsigargin. J Microbiol Biotechnol. 2007;17(7):1134–1138
- Kim HG, Shrestha B, Lim SY, et al. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur J Pharmacol. 2006;545(2–3):192–199
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010;8(5):336–341
- World Health Organization. Definition,diagnosis and classification of diabetes mellitus Report of a WHO consulation, in Geneva World Health Organization. Geneva: World Health Organization; 1999:442–443
- Mogensen CE, Chachati A, Christensen CK, et al. Microalbuminuria: An early marker of renal involvement in diabetes. Uremia Invest. 1985;9(2):85–95
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17(1):1–12
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408–412
- Gao TW, Wei JS. The effect of Jinshuibao Capsule in treatment of 100 patients with diabetic nephropathy. Health Must-Read. 2013;12(3):291
- Song J, Li YH, Yang XD, Guo L, Hu Z. Effect of combined therapy with bailing capsule and benazepril on urinary albumin excretion rate and c-reaction protein in patients with early diabetic nephropathy. Chin J Integr Med. 2009;29(9):791–793
- Xiong J. The effects of bailing capsule on protein marker of renal tubular in patients with diabetic nephropathy. Zhejiang Med J. 2011;33(8):1230–1231
- Zeng SX, Zhang LM. Clinical observation of Irbesartan combined with Jinshuibao Capsule in treatment of type 2 diabetic nephropathy. Chin J Postgrad Med. 2010;33(24):35–37
- Zhang YK. The effections of Irbesartan combined with Jinshuibao capsule on old patients with type 2 diabetic nephropathy. Jilin Med J. 2011;32(12):2342
- Li XL. Clinical effects of losartan combined with bailing capsule in treatment of patients with early diabetic nephropathy. J Tradit Chin Med Inform 2010;2(30):184–185
- Lou PH, Long XS, Li LB. Clinical observation of valsartan combined with bailing capsule in treatment of early diabetic nephropathy. J Pract Med. 2010;26(20):3780–3782
- Cui Y, Li JH, Chen J, et al. The nephroprotection of Angiotensin Converting Enzyme Inhibitors (ACEI) combined with Jinshuibao capsule to diabetic nephropathy patients in clinical research. J Qiqihar Med Coll. 2010;31(14):2256–2257
- Huang T, Sun H, Wu TY. Effect of combined therapy with jinshuiba capsule and perindopril on renal function in old patients with early diabetic nephropathy. Herald Med. 2010;29(7):890–892
- Li H, Nv JN. The nephroprotection of Irbesartan combined with Bailing capsule to Diabetic nephropathy with hypertension patients. China Mod Med. 2011;18(16):56–57
- Li X. Clinical observation of bailing capsule combined with valsartanl in treatment of diabetic nephropathy. China Foreign Med Treat. 2012;28:112–114
- Lin J. The nephroprotection of Valsartan combined with Jinshuibao capsule to diabetic nephropathy. Tianjin Pharm. 2009;21(6):37–39
- Liu YH, Zhao LJ, Wang LH, Gao AM. The influence of Irbesartan combined with Bailing capsule to diabetic nephropathy patients in microalbuminuria. J Chin Physician. 2011;13(2):261–263
- Qian GF, Qin JH. The clinical observation of jinshuibao capsule combined with enalapril in the treatment of diabetic nephropathy. China J Mod Drug Appl. 2012;6(10):96–97
- Shen YL, Chen K. The clinical observation of bailing capsule combined with valsartanl in treatment of diabetic nephropathy. Chin J Pract Med. 2011;38(2):124–125
- Sun QH, Wang J, Liu XL, Zhang WJ, Wang Q. Clinical effect of enalapril combined with Bailing capsule in treatment of old patients with early diabetic kidney disease. Strait Pharm J. 2012;24(10):115–117
- Wang YZ, Zhen LQ. Clinical observation of Benazepril combined with Bailing capsule in treatment of early diabetic nephropathy. J Emerg Tradit Chin Med. 2010;19(5):754–756
- Wei SJ. The clinical effects of Jinshuibao capsule combined with Benazepril in treatment of early type 2 diabetic nephropathy. Heilongjiang Med Pharm. 2010;33(1):50
- Yang JQ, Yang J. Clinical observation of Jinshuibao capsule combined with Benazepril in treatment of early diabetic nephropathy. J Chifeng Univ. 2011;3(2):100–101
- Ye JB, Liu ZM, Li JJ, Lin HZ, Lu J. Clinical observation of Bailing capsule combined with Candesartan cilexetil in treatment of early diabetic nephropathy. Intern Med China. 2012;7(6):612–613
- Yi JZ, Ding GH, Zhu L, Gao Z. The effections of Irbesartan and Jinshuibao on patients with type 2 diabetic kidney disease. J Clin Intern Med. 2009;26(11):741–743
- Yu HT, Shi HT, Xiao LL, Dong CL. The effections of Jinshuibao capsule combined with Olmesartan in treatment of early type 2 diabetic nephropathy. Contemp Med. 2013;19(23):64–65
- Hong YQ. The effections of Irbesartan combined with Bailing capsule on old patients with type 2 diabetic nephropathy. Chin J Misdiagn. 2010;10(24):5857–5858
- Li QY. Clinical observation of Irbesartan combined with Cordyceps sinensis in treatment of type 2 diabetic nephropathy. Contemp Med. 2012;18(22):82–83
- Liu Y. Clinical observation of Telmisartan combined with Jinshuibao capsule in treatment of diabetic kidney disease. China Hosp Pharm J. 2010;30(18):1573–1575
- Shan GH. The clinical observation of Bailing capsule in treatment of early diabetic nephropathy. Chin J Clin Rational Drug Use. 2012;5(11A):81–82
- Tao SC, Zhou QT. The effections of Jinshuibao capsule in treatment of early diabetic nephropathy. J Clin Res. 2009;26(7):1271–1272
- Cao LM. Clinical effects of bailing capsule combined with valsartan in treatment of patients with diabetic nephropathy. Chin J Med Guide. 2012;14(9):1571–1572
- Chen F, Chen BP, Shi J. Clinical observation of Valsartan combined with Bailing capsule in treatment of early diabetic nephropathy. China Pharmacist. 2010;13(4):551–552
- Hong RT. Observation on the treatment of diabetic nephropathy with irbesartan jointed by zhiling capsule. China Modern Doctor. 2008;46(36):96–97
- Liu CP, Li MJ. Effect of combined therapy with irbesartan and bailing capsule on urinary albumin excretion rate and C-reaction protein in patients with early diabetic nephropathy. Hebei Med J. 2011;33(13):1990–1991
- Liu XD. The clinical research of Cordyceps sinensis combined with benazepril in treatment of patients with early diabetic nephropathy. Asia Pacif Tradit Med. 2011;7(12):135–136
- Luo F, Cao S, Sun XY. Clinical research on early diabetic nephropathy treated by bailing capsule combined with Irbesartan. J Chin Med. 2011;25(4):466–467
- Lv ZM. Clinical research of zhiling capsule combined with losartan in treatment of patients with early diabetic nephropathy. Strait Pharm J. 2012;24(2):160–161
- Ma LY, Chen F, Chen BP. Effect of combined therapy with bailing capsule and irbesartan on il-8 in patients with early diabetic nephropathy. China Pharmacist. 2011;14(7):1027–1028
- Qiao AM. The effect of Telmisartan combined with Bailing capsule in treatment of 62 patients with early diabetic nephropathy. China Pract Med. 2013;8(28):177–178
- Shen SM. The clinical observation of valsartan combined with bailing capsule in treatment of 86 patients with early diabetic nephropathy. Chin J Prim Med Pharm. 2012;19(1):127–128
- Wang SY, Wu CF, Zhang H, Shi J, Chen BP. Clinical effects of bailing capsule combined with benazepril in treatment of patients with early diabetic nephropathy. China Pharmacist. 2009;12(4):501–502
- Wang YH, Yuan GF. The clinical observation of bailing capsule combined with telmisartan in treatment of patients with early diabetic nephropathy. J Clin Res. 2008;25(5):927–928
- Yan Y, Wang ZQ. Clinical observation of Bailing Capsule in treatment of patients with early diabetic nephropathy. Chin J Integr Tradit West Nephrol. 2005;6(1):46–47
- Yang XM, Yang KN, Gao W, Cao XJ. Clinical observation of Enalapril combined with Jinshuibao capsule in treatment of diabetic nephropathy. Hebei Med J. 2012;34(6):938–939
- Yu J. Clinical effects and nursing of Cordyceps sinensis combined with losartan in treatment of patients with early diabetic nephropathy. Strait Pharm J. 2012;24(7):184–185
- Zhang Y, Shi HT, Dai HS. The observation on effect of urine protein and lipid metabolism to diabetic nephropathy with Jinshuibao capsules. China Modern Doctor. 2012;50(36):64–65
- Zhen JX. Clinical observation of Irbesartan combined with Bailing capsule in treatment of type 2 diabetic nephropathy. China Mod Med. 2011;18(24):66–67
- Cao XX, Zhang RP, Yang JK. Clinical observation on treatment of diabetic nephropathy with Jinshuibao capsules and valsartan. Chin J New Drugs. 2007;16(16):1303–1306
- Lei SH, Li J, Lai XY, et al. Clinical observation of Irbesartan combined with Jinshuibao capsule in treatment of diabetic nephropathy. Shandong Med J. 2009;49(1):98–99
- Lu YL. Clinical observation of Telmisartan combined with Bailing capsule in treatment of diabetic nephropathy. Chin J Clin Rational Drug Use. 2010;3(21):43–44
- Shi HB. Clinical observation of Perindopril combined with Jinshuibao capsule in treatment of type 2 diabetic nephropathy. Chin J Pract Med. 2010;37(7):46–47
- Wang HP. Clinical observation of Fosinopril combined with Jinshuibao capsule in treatment of type 2 diabetic nephropathy. Tianjin Pharmacy. 2007;19(3):27–28
- Xue XH. Clinical observation of Irbesartan combined with Bailing capsule in treatment of type 2 diabetic nephropathy. Shanxi Med J. 2009;38(suppl):45–46
- Yang CH. Clinical observation of Jinshuibao capsule combined with losartan in treatment of early diabetic nephropathy. Mod J Integr Tradit Chin West Med. 2013;22(1):65–66
- Zhang ZF, Lv J. Clinical observation of bailing capsule in treatment of patients with early diabetic nephropathy. Shanxi Med J. 2009;38(8):730–731
- Zhou P. Clinical observation of losartan potassium with Jinshuibao capsule in patients with early type 2 diabetic nephropathy. Xinjiang Med J. 2012;42:21–23
- Chen WJ. Observation of the effect of Zhiling capsule combined with irbesartan treating early diabetic nephropathy. Mod Hospital. 2011;11(8):57–58
- Guo JZ, Yan HY. Clinical observation of irbesartan combined with Jinshuibao capsule in treatment of patients with early type 2 diabetic nephropathy. Ningxia Med J. 2012;34(9):931–932
- Liu MW. Clinical effects of losartan combined with bailing capsule in treatment of patients with diabetic nephropathy. Chin J Misdiagn. 2011;11(26):6336
- Wang L, Su XF. Effect of combined therapy with bailing capsule and losartan on microalbuminuria in patients with early type 2 diabetic nephropathy. Chin J Gerontol. 2007;27:972–973
- Wu WT. Effect analysis of valsartan and cordyceps preparation in the treatment of type 2 diabetic nephropathy. China Mod Med. 2012;19(27):71–72
- Fang YY. Clinical observation of Telmisartan combined with Jinshuibao capsule in treatment of early diabetic nephropathy. J Clin Res. 2010;27(10):1907–1908
- Guan HB, He KP, Huan WM, et al. Effect of Irbesartan with bailing capsules on microalbuminuria-to-creatinine ratio and high-sensitive C-reactive protein in patients with early diabetic nephropathy. Chin Gen Pract. 2010;13(9B):2934–2936
- Kashihara N, Haruna Y, Kondeti VK, Kanwar YS. Oxidative stress in diabetic nephropathy. Curr Med Chem. 2010;17(34):4256–4269
- Tang WH, Cheng WT, Kravtsov GM, et al. Cardiac contractile dysfunction during acute hyperglycemia due to impairment of SERCA by polyol pathway-mediated oxidative stress. Am J Physiol Cell Physiol. 2010;299(3):C643–C653
- Thallas-Bonke V, Thorpe SR, Coughlan MT, et al. Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes. 2008;57(2):460–469
- Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm. 2012;2012:146154
- Bohlender JM, Franke S, Stein G, Wolf G. Advanced glycation end products and the kidney. Am J Physiol Renal Physiol. 2005;289(4):F645–F659
- Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M, Garcia-Perez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7(6):327–340
- Singh DK, Winocour P, Farrington K. Oxidative stress in early diabetic nephropathy: Fueling the fire. Nat Rev Endocrinol. 2011;7(3):176–184
- Liu ZY, Yao YJ, Liang ZQ, et al. Molecular evidence for the anamorph-teleomorph connection in Cordyceps sinensis. Mycol Res. 2001;105:827–832
- Wang J, Liu YM, Cao W, et al. Anti-inflammation and antioxidant effect of Cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis, in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Metab Brain Dis. 2012;27(2):159–165
- Dworecka-Kaszak B. Cordyceps fungi as natural killers, new hopes for medicine and biological control factors. Ann Parasitol. 2014;60(3):151–158
- Yu HM, Wang BS, Huang SC, Duh PD. Comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. J Agric Food Chem. 2006;54(8):3132–3138
- Xiao L, Ge Y, Sun L, et al. Cordycepin inhibits albumin-induced epithelial-mesenchymal transition of renal tubular epithelial cells by reducing reactive oxygen species production. Free Radic Res. 2012;46(2):174–183
- Wang ZJ, Wang SC. Research progress in the mechanism of Cordyceps sinensis in diabetic nephropathy. Chin J Integr Med. 2008;9(1):88–90
- Yan JK, Li L, Wang ZM, et al. Acidic degradation and enhanced antioxidant activities of exopolysaccharides from Cordyceps sinensis mycelial culture. Food Chem. 2009;117:614–616
- Choi JW, Ra KS, Kim SY, et al. Enhancement of anti-complementary and radical scavenging activities in the submerged culture of Cordyceps sinensis by addition of citrus peel. Bioresour Technol. 2010;101(15):6028–6034
- Rathor R, Mishra KP, Pal M, et al. Scientific validation of the Chinese caterpillar medicinal mushroom, Ophiocordyceps sinensis (Ascomycetes) from India: Immunomodulatory and antioxidant activity. Int J Med Mushrooms. 2014;16(6):541–553
- Dong C, Yao Y. Isolation, characterization of melanin derived from Ophiocordyceps sinensis, an entomogenous fungus endemic to the Tibetan Plateau. J Biosci Bioeng. 2012;113(4):474–479
- Yang L, Guo J. The influence of bailing capsule on oxidative stress in early diabetic nephropathy. Chin J Gerontol. 2012;16:3518–3520
- Chung SS, Chung SK. Aldose reductase in diabetic microvascular complications. Curr Drug Targets. 2005;6(4): 475–486
- Deng YY, Chen YP, He XL, Li L. Study of Cordyceps on mechanism in delaying chronic renal failure. Chin J Integr Tradit West Nephrol. 2001;2(7):381–383
- Hauser PV, Collino F, Bussolati B, Camussi G. Nephrin and endothelial injury. Curr Opin Nephrol Hypertens. 2009;18(1):3–8
- Chang QT, Fang JA. Effect of bailing capsule on the expression of nephrin in the kidney of the rats with diabetic nephropathy. Chin J Integr Med. 2010;11(1):10–13
- Ihn H. Pathogenesis of fibrosis: Role of TGF-beta and CTGF. Curr Opin Rheumatol. 2002;14(6):681–685
- Gu L, Johno H, Nakajima S, et al. Blockade of Smad signaling by 3'-deoxyadenosine: A mechanism for its anti-fibrotic potential. Lab Invest. 2013;93(4):450–461
- Li L, He D, Yang J, Wang X. Cordycepin inhibits renal interstitial myofibroblast activation probably by inducing hepatocyte growth factor expression. J Pharmacol Sci. 2011;117(4):286–294
- Fang JA, Deng GA. Experimental study on the protective effect of bailing capsule on diabetic nephropathy in rats. PhD Thesis. TongJi Medical College, HUST, Wuhan, China; 2005
- Thomson DM, Winder WW. AMP-activated protein kinase control of fat metabolism in skeletal muscle. Acta Physiol (Oxf). 2009;196(1):147–154
- Kiho T, Yamane A, Hui J, Usui S, Ukai S. Polysaccharides in fungi. XXXVI. Hypoglycemic activity of a polysaccharide (CS-F30) from the cultural mycelium of Cordyceps sinensis and its effect on glucose metabolism in mouse liver. Biol Pharm Bull. 1996;19(2):294–296
- Yamaguchi Y, Kagota S, Nakamura K, Shinozuka K, Kunitomo M. Antioxidant activity of the extracts from fruiting bodies of cultured Cordyceps sinensis. Phytother Res. 2000;14(8):647–649
- Yuan M, Tang R, Zhou Q, et al. Effect of Cordyceps sinensis on expressions of HIF-1alpha and VEGF in the kidney of rats with diabetic nephropathy. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2013;38(5):448–457
- Tu S, Zhou QL, Tang R, et al. Proapoptotic effect of angiotensin II on renal tubular epithelial cells and protective effect of Cordyceps sinensis. J Cent South Univ(Med Sci). 2012;37(1):67–72
- Shen SJ. Bailing protect against tubular injury in nephrotic syndrome. Zhejiang Med J. 2002;24(8):497–498