Abstract
Objective: The aim of this study was to determine the effect of oral cholecalciferol treatment on vascular calcification, left ventricular mass index (LVMI) and other cardiac functions in dialysis patients. Design and methods: A six-month course of oral cholecalciferol treatment was recommended to dialysis patients with vitamin D insufficiency. While 26 patients were given cholecalciferol treatment, 17 patients who could not tolerate to therapy received standard therapy. Initial biochemical parameters were measured, and they were measured again after 6 months of treatment. Echocardiographic measurements were also performed, and the vascular calcification score (VCS) was calculated at baseline and at the 6th month. Results: The cholecalciferol replacement group showed no significant change in LVMI and VCS values (p > 0.05). However, while LVMI was similar between groups at initial evaluation, it was lower in the cholecalciferol group at the 6th month when compared to the standard treatment group (141.8 ± 40.2 g/m2 vs. 166.3 ± 31.4 g/m2; p = 0.04). Likewise, left ventricular diastolic diameters (48.8 ± 5.1 mm vs. 47.5 ± 4.6 mm; p = 0.023) and left atrial diameters (41.2 ± 8.9 mm vs. 38.9 ± 8.1 mm; p = 0.006) decreased in the cholecalciferol group. Additionally, significant increases were observed in serum 25-hydroxyvitamin D (25(OH)D) and albumin levels, with a significant decrease in serum C-reactive protein levels. Conclusion: A lesser increase in left ventricular mass and better diastolic functions was observed in dialysis patients after 6 months of cholecalciferol treatment.
Introduction
The leading cause of mortality in patients with chronic kidney disease (CKD) is cardiovascular events.Citation1 In these patients, left ventricular hypertrophy (LVH) and vascular calcification play an important role in the development of cardiovascular diseases.Citation2,Citation3 A majority of patients with CKD have vitamin D insufficiency, whose effect on the cardiovascular and musculoskeletal systems, among others, is well known.Citation4–7 In observational studies, low vitamin D levels have been shown to be associated with increased cardiovascular morbidity and mortality.Citation8–10
Recently, localized production of calcitriol, which is involved in modulation of cellular growth and differentiation of epithelial cells, has been shown in other cell types. It is assumed that combination of a calcitriol analog with vitamin D (cholecalciferol or ergocalciferol) is required to ensure that vitamin D deficiency is also corrected.Citation11,Citation12
In addition, it has been demonstrated in rat models that treatment of vitamin D deficiency reduces LVH, and enhances diastolic functions.Citation13–16 Prospective studies have demonstrated that cholecalciferol treatment reduces left ventricular mass index (LVMI),Citation17,Citation18 but these are conflicted because in recent randomized controlled trial, cholecalciferol treatment did not change LVMI.Citation19
The aim of the present study was to evaluate the effect of oral cholecalciferol treatment on vascular calcification, left ventricular mass index and other cardiac functions in dialysis patients with stage 5 CKD.
Materials and methods
Study population and study design
Forty-three of 55 patients over 18 years of age who were on dialysis treatment for more than 3 months were enrolled in this study, while 12 patients were excluded. The exclusion criteria were acute infection, chronic inflammation, malignancy, chronic diarrhea, intestinal malabsorption, moderate-severe aortic stenosis and amyloidosis.
The study comprised dialysis patients followed by the Nephrology Department of Süleyman Demirel University Hospital with serum 25-hydroxyvitamin D (25(OH)D) levels <30 ng/mL. Patients who did not tolerate or did not adhere to cholecalciferol treatment were assigned to the standard treatment group while the rest were recruited as the cholecalciferol group. Baseline and 6th month measurements of serum 25(OH)D levels were evaluated, along with routine biochemical tests, echocardiographic data and vascular calcification scores. Additionally, demographic data, and data on primary renal disease, overall dialysis duration, accompanying disease and medication were obtained from hospital records. Informed consent was sought from all participants, and the study was approved by Süleyman Demirel University Faculty of Medicine, Ethics Committee of Clinical Trials (2013/38).
Definition of vitamin D insufficiency and cholecalciferol usage
Serum 25(OH)D levels below 30 ng/mL were defined as insufficiency of vitamin D. Oral cholecalciferol (Devit3®) treatment was arranged as 50,000 IU/week for the first 3 months and 10,000 IU/week for the following 3 months for patients with serum 25(OH)D level < 15 ng/mL. As recommended by Garcia-Lopes et al., it was prescribed as 10,000 IU/week for 6 months for patients with serum 25(OH)D levels of 15–30 ng/mL.Citation20 All patients with secondary hyperparathyroidism were treated with calcitriol or cinacalcet according to the Kidney Disease: Improving Global Outcomes (KDIGO) guideline of 2009.Citation21
Laboratory analysis
Serum calcium (Ca), phosphate (P), albumin, triglyceride, total cholesterol, LDL-cholesterol, HDL-cholesterol, intact parathormone (iPTH), 25(OH)D, ferritin, C-reactive protein (CRP) and total blood counts were measured at the baseline and again 6 months after treatment. Total blood count measurements were performed by impedance method, using the Beckman Coulter LH780 Analyzer (Beckman Coulter Inc., Brea, CA) autoanalyzer; biochemical tests by the spectrophotometric method, using the Beckman Coulter AU5800 (Beckman Coulter Inc., Brea, CA) autoanalyzer; CRP by the nephelometric method, using the Beckman Coulter Image (Beckman Coulter Inc., Brea, CA) device and iPTH and 25(OH)D by the chemiluminescence method, using the Beckman Coulter UnıCel DxI 800 (Beckman Coulter Inc., Brea, CA) device. Serum calcium levels were corrected by serum albumin levels. Kt/V values were calculated for all patients.
Echocardiographic evaluation
Transthoracic echocardiographic evaluation was performed for all participants in accordance with the recommendations of the American Echocardiography Society.Citation22 The images were obtained in supine position at 30 ° tilt, using the Philips HD15 (Philips Medical Systems, Bothell, WA) echocardiography device and a 1.2–4.3 MHz S2 probe. Two-dimensional and M-mode measurements were performed along with apical four-chamber, two-chamber and three-chamber views, all by the same researcher.
The diameter and volume of the left atrium, and diameter of the left ventricle and ejection fraction (LVEF) were measured. LVMI and vascular calcification scores were calculated using the Devereux formula.Citation23 The E/A ratio was calculated for diastolic functions of the left ventricle by measuring the velocity of transmitral E and A waves. E/E′ ratio of the mitral valve was calculated by tissue Doppler sonography.
Vascular calcifications
Evaluation of vascular calcifications was performed by the same researcher using the scoring system defined by Adragao et al.Citation24 for X-ray images. Posteroanterior hand and pelvis graphs were performed on all participants; calcifications of the radial and digital arteries on hand graph and iliac and femoral arteries on pelvis graph were investigated. Each area was scored “0” for absence of vascular calcification and “1” for its presence, yielding a score of “0–8” points of vascular calcification for each patient.
Statistical analysis
Statistical Package for Social Sciences (SPSS) software version 15.0 for Windows (SPSS Inc., Chicago, IL) was used to analyze the data. Continuous variables were presented as mean and standard deviation, and categorical variables were presented as percentages. Demographic and laboratory data were compared at baseline and 6 months. Student's t-test was used to compare continuous variables between the two groups, and the chi-square test was used to compare categorical variables. Paired sample t-testing was used in the evaluation of data before and after treatment; p-value less than 0.05 was considered statistically significant.
Results
Baseline characteristics
Mean age of the 43 participants was 55.4 ± 14.9 years. Twenty of the patients (46%) were male. Twenty-two patients were on peritoneal dialysis, and 21 were on hemodialysis. The calcium content of the dialysis solutions was 1.25 mmol/L. Mean dialysis treatment duration was 52.1 ± 56.3 months. The etiologies of chronic renal disease were hypertension in 51% of the patients, diabetes mellitus in 23%, chronic glomerulonephritis in 12% and chronic pyelonephritis in 5%. The etiology of the remaining 9% was unknown. Six patients had a history of coronary artery disease, and seven patients had a history of cardiac failure. Eighty-eight percent of the patients had LVH, and 50% had vascular calcification. Baseline demographic data and, clinical, laboratory and echocardiographic findings for cholecalciferol and standard treatment groups are set out in . Baseline characteristics of the groups were similar.
Table 1. Baseline characteristics of study groups.
Cholecalciferol group
Serum 25(OH)D levels of 73% of the cholecalciferol group increased to normal range after treatment. None of the levels were toxic for patients.
Significant decreases in left ventricular diameter (41.2 ± 8.9 mm vs. 38.9 ± 8.1 mm; p = 0.006) and left ventricular diastolic diameter (48.8 ± 5.1 mm vs. 47.5 ± 4.6 mm; p = 0.023) were observed. However, significant changes in LVMI and vascular calcification scores were not found (p > 0.05, ).
Table 2. Baseline and sixth month evaluations of cholecalciferol treatment group (n = 26).
When baseline and 6th month laboratory analyses of the cholecalciferol group were taken into consideration, a significant increase was detected in serum albumin levels (3.5 ± 0.5 g/dL vs. 3.7 ± 0.5 g/dL; p = 0.03) with significant decrease in CRP levels (7.3 ± 4.6 g/dL vs. 5.5 ± 2.8 g/dL; p = 0.014). No significant changes were observed in serum Ca, P, iPTH and hemoglobin levels (p > 0.05, ).
Intergroup comparisons
While baseline LVMI values were similar for the cholecalciferol and standard treatment groups, LVMI of the cholecalciferol group was lower at the 6th month of treatment (141.8 ± 40.2 g/m2 vs. 166.3 ± 31.4 g/m2, p = 0.04, ).
Figure 1. Comparison of left ventricular mass index (LVMI) values of study groups. These values were compared between standard treatment and cholecalciferol groups with Student's t-test. Note: Values (g/m2) are expressed as mean ± SD.
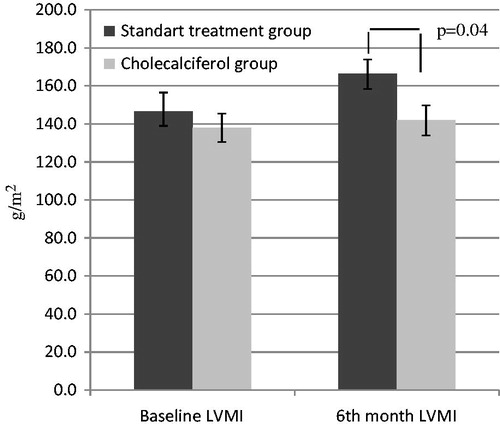
When compared to the standard treatment group, there were no significant changes in the cholecalciferol group in terms of serum P, iPTH, albumin, hemoglobin, CRP, LVMI and vascular calcification score, but calcium levels increased (p = 0.01, ) and E/E′ decreased (p = 0.007, ). In addition left atrial and left ventricular diastolic diameters reduced slightly in the cholecalciferol group (p = 0.05 and p = 0.06, ).
Table 3. Comparison of baseline and sixth month evaluations of study groups.
Discussion
The present study revealed that oral cholecalciferol treatment for dialysis patients with vitamin D insufficiency decelerated but did not reverse the progression of LVH; enhancement of diastolic functions, nutritional status and inflammation was also observed. Vitamin D insufficiency, frequency of LVH and vascular calcification were found to be higher in dialysis patients. No significant alterations were observed in serum Ca and P levels or vascular calcification scores after cholecalciferol replacement.
After 6 months of oral cholecalciferol replacement, Matias et al. detected a significant decrease in LVMI of dialysis patients at the 12th month.Citation17 Similarly, Bucharles et al. observed a significant decrease in LVMI after 6 months of oral cholecalciferol treatment in hemodialysis patients with vitamin D insufficiency that did not have hyperparathyroidism.Citation18 The present study demonstrated that oral cholecalciferol treatment did not regress LVMI but decelerated its progression significantly by comparison with the standard treatment group. The mean overall dialysis duration of these patients was longer than the dialysis duration in both previous studies, which may explain why LVMI did not regress significantly after cholecalciferol treatment. Additionally, the lack of control groups was acknowledged as a limitation of those two studies.
In a 6-month placebo controlled randomized study of 3000 IU/day cholecalciferol treatment on dialysis patients, Mose et al. observed no enhancement in LVMI and cardiac functions.Citation19 This finding may have resulted from administration of low-dose cholecalciferol.
Left atrial enlargement and increased left ventricular mass are related to increase in cardiovascular risk.Citation25,Citation26 Animal studies have shown that vitamin D treatment enhances left ventricular diastolic functions.Citation13,Citation16 In a study by Tamez et Al. on stage 2–4 CKD patients with LVH, it was shown that 48 weeks of oral paricalcitol treatment reduced left atrial volume index but did not affect other echocardiographic parameters.Citation27 In the present study, significant decrease was detected in the diastolic diameters of left atrium and left ventricle after cholecalciferol treatment.
There is conflicting evidence concerning the effect of vitamin D on vascular calcification. It has been reported that high-dose usage of vitamin D analogues may increase vascular calcifications,Citation28 but claims have also been made of a negative correlation between serum 25(OH)D levels and vascular calcification.Citation29 In a placebo controlled randomized study on hemodialysis patients, Delanaye et al. stated that cholecalciferol treatment did not alter vascular calcification scores at the end of a one-year period.Citation30 In the present study, vascular calcification scores also remained unchanged, although here-cholecalciferol was administered in higher doses and for a shorter period. From these results, it may be assumed that cholecalciferol treatment over a six-month period would not increase vascular calcification in patients whose Ca and P metabolisms are under control.
Bucharles et al. observed, a significant decrease in high-sensitivity-CRP and IL-6 levels after 6 months of oral cholecalciferol treatment.Citation18 Matias et al. also found that CRP levels decreased significantly.Citation17 These findings are supported in the present study, as there was a significant decrease in CRP levels, which may be explained by the anti-inflammatory effects of vitamin D.
In their study on hemodialysis patients, Matias et al. detected a positive correlation between serum 25(OH)D levels and albumin, which they attributed to malnutrition, accompanied by insufficient vitamin intake and reduced exposure to daylight.Citation31 It has been reported that cholecalciferol treatment increases serum albumin levels of dialysis patients,Citation17,Citation32 and a small increase in serum albumin level after cholecalciferol treatment was also observed in the present study. However, this increment was not statistically significant when compared to the standard treatment group.
A relationship has previously been reported between malnutrition–inflammation syndrome and endothelial dysfunction in hemodialysis and peritoneal dialysis patients.Citation33,Citation34 The effect of cholecalciferol treatment on malnutrition–inflammation syndrome in dialysis patients is unknown. In the present study, a positive effect on nutrition and inflammation was observed for cholecalciferol treatment. Randomized controlled trials are required to clarify this issue.
The limitations of the present study are the lack of randomization, lack of statistical power analysis, relatively small patient numbers and a short follow-up period. The strengths of this study include the use of a control group with similar characteristics to the patient group, evaluation of vascular calcifications, and its contribution to the literature as the first study to evaluate the addition of cholecalciferol to standard treatment.
In summary, six months of oral cholecalciferol treatment decelerates the increase in left ventricular mass, reduces inflammation and enhances diastolic functions. However, the treatment seems to be ineffective in respect of vascular calcification. For greater certainty, long-term randomized controlled studies are required.
Declaration of interest
All authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S112–S119
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events and hospitalization. N Engl J Med. 2004;351(13):1296–1305
- Goodman WG, London G, Amann K, et al. Vascular calcification in chronic kidney disease. Am J Kidney Dis. 2004;43(3):572–579
- Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118(12):3820–3828
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281
- Autier P, Gandini S. Vitamin D supplementation and total mortality: A meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167(16):1730–1737
- Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511
- Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25OHD and risk of myocardial infarction in men: A prospective study. Arch Intern Med. 2008;168:1174–1180
- Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all cause and cardiovascular mortality. Arch Intern Med. 2008;168(12):1340–1349
- Ginde AA, Scragg R, Schwartz RS, Camargo CA Jr. Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older US adults. J Am Geriatr Soc. 2009;57(9):1595–1603
- Jones G. Why dialysis patients need combination therapy with both cholecalciferol and a calcitriol analogs. Semin Dial. 2010;23(3):239–243
- Dusilová-Sulková S, Safránek R, Vávrová J, Horáček J, Pavlíková L, Palička V. Low-dose cholecalciferol supplementation and dual vitamin D therapy in hemodialysis patients. Int Urol Nephrol. 2015;47(1):169–176
- Bodyak N, Ayus JC, Achinger S, et al. Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci USA. 2007;104(43):16810–16815
- Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol. 2007;103(3-5):521–524
- Przybylski R, McCune S, Hollis B, Simpson RU. Vitamin D deficiency in the spontaneously hypertensive heart failure (SHHF) prone rat. Nutr Metab Cardiovasc Dis. 2010;20(9):641–646
- Bae S, Yalamarti B, Ke Q, et al. Preventing progression of cardiac hypertrophy and development of heart failure by paricalcitol therapy in rats. Cardiovasc Res. 2011;91(4):632–639
- Matias PJ, Jorge C, Ferreira C, et al. Cholecalciferol supplementation in hemodialysis patients: Effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol. 2010;5(5):905–911
- Bucharles S, Barberato SH, Stinghen AE, et al. Impact of cholecalciferol treatment on biomarkers of inflammation and myocardial structure in hemodialysis patients without hyperparathyroidism. J Ren Nutr. 2012;22(2):284–291
- Mose FH, Vase H, Larsen T, et al. Cardiovascular effects of cholecalciferol treatment in dialysis patients – A randomized controlled trial. BMC Nephrol. 2014;15:50
- Garcia-Lopes MG, Pillar R, Kamimura MA, et al. Cholecalciferol supplementation in chronic kidney disease: Restoration of vitamin D status and impact on parathyroid hormone. Ann Nutr Metab. 2012;61(1):74–82
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD–MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD–MBD). Kidney Int Suppl. 2009;(113):S1–S130
- Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2(5):358–367
- Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1986;57(6):450–458
- Adragao T, Pires A, Lucas C, et al. A simple vascular calcification score predicts cardiovascular risk in hemodialysis patients. Nephrol Dial Transplant. 2004;19(6):1480–1488
- Miller JT, O'Rourke RA, Crawford MH. Left atrial enlargement: An early sign of hypertensive heart disease. Am Heart J. 1988;116(4):1048–1051
- Ristow B, Ali S, Whooley MA, Schiller NB. Usefulness of left atrial volüme index to predict heart failure hospitalization and mortality in ambulatory patients with coronary heart disease and comparison to left ventricular ejection fraction (from the Heart and Soul Study). Am J Cardiol. 2008;102(1):70–76
- Tamez H, Zoccali C, Packham D, et al. Vitamin D reduces left atrial volume in patients with left ventricular hypertrophy and chronic kidney disease. Am Heart J. 2012;164(6):902–909.e2
- Cardús A, Panizo S, Parisi E, Fernandez E, Valdivielso JM. Differential effects of vitamin D analogs on vascular calcification. J Bone Miner Res. 2007;22(6):860–866
- García-Canton C, Bosch E, Ramírez A, et al. Vascular calcification and 25-hydroxyvitamin D levels in non-dialysis patients with chronic kidney disease stages 4 and 5. Nephrol Dial Transplant. 2011;26(7):2250–2256
- Delanaye P, Weekers L, Warling X, et al. Cholecalciferol in hemodialysis patients: A randomized, double-blind, proof-of-concept and safety study. Nephrol Dial Transplant. 2013;28(7):1779–1786
- Matias PJ, Ferreira C, Jorge C, et al. 25-Hydroxyvitamin D3, arterial calcifications and cardiovascular risk markers in hemodialysis patients. Nephrol Dial Transplant. 2009;24(2):611–618
- Jean G, Terrat JC, Vanel T, et al. Daily oral 25-hydroxycholecalciferol supplementation for vitamin D deficiency in hemodialysis patients: effects on mineral metabolism and bone markers. Nephrol Dial Transplant. 2008;23(11):3670–3676
- Aguilera A, Sánchez-Tomero JA, Bajo MA, et al. Malnutrition–inflammation syndrome is associated with endothelial dysfunction in peritoneal dialysis patients. Adv Perit Dial. 2003;19:240–245
- Demir M, Kucuk A, Sezer MT, Altuntas A, Kaya S. Malnutrition–inflammation score and endothelial dysfunction in hemodialysis patients. J Ren Nutr. 2010;20(6):377–383

