Abstract
Transforming-growth factor β1 (TGF-β1) is a powerful cytokine involved in physiological processes of growth, differentiation, gene expression, embryogenesis, tissue remodelling, wound healing as well as tumorigenesis, immunosuppression and fibrosis, like peritoneal membrane fibrosis on long-term peritoneal dialysis (PD) treatment. The aims of this study were to determine TGF-β1 levels in serum (s) and drained dialysate (dd), to assess their relations to sex, age, diabetes, dialysis modality, peritonitis and use of erythropoiesis stimulating agents (ESAs), inhibitors of angiotensin-converting enzyme (ACEi) and/or statins in 20 patients, 11 men and 9 women, mean age 62.90 ± 12.69 years, free of peritonitis during the first 6 months of PD treatment. There was no statistically significant difference in TGF-β1 concentrations in serum and drained dialysate at the beginning and after first 6 months of chronic PD, in patients of different sex, age and diabetic patients versus non-diabetic. The significant positive correlations between sTGF-β1 levels and glycemia at the beginning and cholesterolemia after 6 months of PD treatment suggest higher TGF-β1 concentrations in patients with unfavorable metabolic profile. Expression of TGF-β1 in effluent dialysate was significantly lower in patients on chronic PD using ACEi therapy, suggesting ACEi to have a protective effect on peritoneal membrane. Patients on ESA had slightly lower sTGF-β1 concentrations after the first 6 months of PD treatment.
Introduction
Transforming-growth factor β (TGF-β) is a member of the transforming growth factor beta superfamily, which includes at least three isoforms of TGF-β (1, 2 and 3), inhibins, activin, anti-müllerian hormone, bone morphogenetic protein, decapentaplegic and Vg-1. It is secreted by macrophages and many other cell types in a latent form in which it is complexed with two other polypeptides, latent TGF-β binding protein and latency-associated peptide. Serum proteinases, such as plasmin, catalyze the release of active TGF-β from the complex.Citation1 This often occurs on the surface of macrophages where the latent TGF-β complex is bound to CD36 via its ligand, thrombospondin-1. Inflammatory stimuli that activate macrophages enhance the release of active TGF-β by promoting the activation of plasmin. Macrophages can also uptake by endocytosis IgG-bound latent TGF-β complexes that are secreted by plasma cells and then release active TGF-β into the extracellular fluid.Citation2
TGF-β controls cellular growth, proliferation, differentiation, apoptosis and other functions in most cells. It plays a major role in immunity, cancer, bronchial asthma, heart disease, hereditary hemorrhagic telangiectasia, Marfan syndrome, vascular Ehlers–Danlos syndrome, Loeys–Dietz syndrome, Parkinson's disease and AIDS.Citation2,Citation3 TGF-β is important in regulating glucose and energy homeostasis and plays a role in the development of diabetic nephropathy.Citation1,Citation3
Increased concentration of TGF-β1 may be a biomarker of the development of cardiovascular diseases, as well as a prognostic factor in the evaluation of atherosclerosis progression in patients affected by end-stage renal disease and treated with peritoneal dialysis, a widely used modality of renal replacement therapy during the last decades.Citation4 TGF-β1 plays a specific role in the development of peritoneal membrane fibrosis on long-term peritoneal dialysis treatment: it is secreted in mesothelial cells cultures and it up-regulates synthesis of collagen and fibronectin in the peritoneum, and actin in α-smooth muscle cells.Citation5,Citation6 Conventional peritoneal dialysis fluids with high glucose concentration up-regulate fibronectin and TGF-β1 and down-regulate perlecan in peritoneal mesothelial cells cultures.Citation7,Citation8
Aim of the study
The aims of this study were to determine TGF-β1 levels in serum and drained dialysate and to assess their relations to sex, age, diabetes, dialysis modality, peritonitis and use of erythropoiesis stimulating agents (ESAs), inhibitors of angiotensin-converting enzyme (ACEi) and/or statins in end-stage renal failure patients during the first 6 months of peritoneal dialysis treatment.
Materials and methods
Patients and methods
Patients
The study included 20 patients (11 men and 9 women, mean age 62.90 ± 12.69 years, seven patients were older then 65) followed during the first 6 months of chronic peritoneal dialysis treatment. Most patients, 12 of them, were on continuous ambulatory peritoneal dialysis (CAPD), six were on continuous cycling peritoneal dialysis (CCPD) and two on automated peritoneal dialysis (APD). All patients were free of peritonitis, clinical and/or laboratory signs of other infection during the 4 weeks prior to the enrollment. The leading cause of end-stage renal failure was diabetes mellitus in 11 patients, hypertensive nephroangiosclerosis in three patients, chronic glomerulonephritis in one patient, obstructive nephropathy in one patient and unknown renal disease in four patients. The study protocol was reviewed and approved by the Ethical Committee, School of Medicine, University of Belgrade. All patients gave informed consent to participate in the study.
Data concerning use of ACEi and statins were collected from medical records or during regular medical visits in the outpatient unit. Arterial blood pressure measurements and cholesterol blood level assessments indirectly provided insight in patients' degree of compliance with therapy. Patients receiving ACEi were treated with enalapril or fosinopril, while those on lipid lowering therapy used simvastatin or atorvastatin. Erythropoiesis stimulating agents were applied in the outpatient unit. Three patients were treated with epoetin-beta and nine patients with darbepoetin-alfa, in doses adjusted to target hemoglobin level 110 g/L. Data concerning peritonitis occurrence were collected from medical records. In our group three patients experienced one episode of peritonitis each (one caused by Staphylococcus coagulase negative, and the other two cultures were sterile, all treated with cephalosporins during 14 days) and the others were free of peritonitis during the 6 months follow up.
Biochemical analysis
Morning samples of blood and overnight drained dialysate were taken and analyzed at the beginning and after 6 months of peritoneal dialysis treatment. Hemoglobin, hematocrit, serum iron, ferritin, total iron binding capacity (TIBC), percentage of iron saturation, glucose, urea, creatinine, total protein (TP), albumin, total cholesterol, triglycerides, intact parathyroid hormone (iPTH), fibrinogen and C-reactive protein (CRP) levels were assessed in blood samples. Levels of TGF-β1 were assessed in serum and drained dialysate samples.
Complete blood count was determined from blood samples taken in Vacutainer vials with K3EDTA anticoagulant with the Beckman Coulter® HmX Hematology Analyzer (Abbott Diagnostics, Wiesbaden, Germany). Hemoglobin was determined with cyanmethemoglobin method.
Blood samples for biochemical analyses were taken in biochemical vials, then centrifuged at 3000 rpm for 10 min and analyzed with the ARCHITECT ci8200 (Abbott Diagnostics, Wiesbaden, Germany) analyzer.
The iPTH levels were assessed on Architect Intact (Abbott Diagnostics, Abbott Park, IL), by two site sandwich immunoassay using direct chemiluminometric technology.
The plasma fibrinogen concentration was assessed using analyzer BCS® System SIMENS (SIMENS, Muenchen, Germany) by the modified Clauss method using Siemens Healthcare Diagnostics' Multifibren U Reagent (serum fibrinogen referent levels 2.1–4.0 g/L).
We used the sandwich type enzyme-linked immunosorbent assay (ELISA) test Quantikine® Human TGF-β1, R&D Systems, USA & Canada, to detect TGF-β1 in serum and drained dialysate. The micro-ELISA plate provided in this kit has been pre-coated with a monoclonal antibody specific to the TGF-β1 antigen. Standards or samples were added to the appropriate micro ELISA plate wells and combined with the specific antibody. Then a biotinylated detection antibody specific for the antigen and Avidin-Horseradish Peroxidase (HRP) conjugate were added to each micro-plate well successively and incubated. Free components were washed away. The substrate solution was added to each well. Only those wells containing the antigen, biotinylated detection antibody and Avidin-HRP conjugate appeared blue in color. The enzyme–substrate reaction was terminated by the addition of a sulphuric acid solution and the color turned yellow. The optical density (OD) was measured spectrophotometrically at a wavelength of 450 nm ± 2 nm. The OD value was proportional to the concentration of the antigen. The concentration of antigen in the samples was calculated by comparing the OD of the samples to the standard curve. The reference range is 18.289–63.416 pg/mL for TGF-β1 in serum and undetectable to 257 pg/mL for TGF-β1 in urine. So far there are no reference values set for drained dialysate TGF-β1 concentration.
Statistical analysis
Results are shown as mean ± standard deviation (SD) or median where appropriate. Pearson chi-square test and Student's t-test were used to compare groups. Pearson and Spearman tests were used to measure the degree of correlation between variables. Analysis was performed with SPSS 20.0 (IBM Corp, Armonk, NY). All p values less than 0.05 were considered significant.
Results
At the beginning of chronic peritoneal dialysis treatment patients' average body height was 170.6 ± 7.96 cm (range 158–185 cm), body mass 69.99 ± 8.97 kg (range 52–82 kg), body mass index (BMI) 24.03 ± 2.52 (range 17.97–29.38), volume of body water 36.81 ± 5.12 L (range 27.62–43.96 L) and body surface area 1.81 ± 0.15 m2 (range 1.51–2.05 m2).
No statistically significant difference was observed between TGF-β1 levels at baseline in men versus women in serum (p = 0.227) and drained dialysate (p = 0.508), younger versus elderly patients in serum (p = 0.390) and in drained dialysate (p = 0.767), diabetic versus non-diabetic patients in serum (p = 0.263) and in drained dialysate (p = 0.349).
At baseline the serum TGF-β1 concentrations were 15.43 ng/mL ± 1.52 ng/mL in patients on APD, 24.73 ng/mL ± 11.8 ng/mL in patients on CAPD, 34.8 ng/mL ±24.53 ng/mL in patients on CCPD and drained dialysate TGF-β1 concentrations were in patients on APD 0.65 ng/mL ± 0 ng/mL, in patients on CAPD 1.02 ng/mL ±1.07 ng/mL, in patients on CCPD 0.75 ng/mL ± 0.24 ng/mL. Serum and drained dialysate TGF-β1 concentrations were not significantly different in patients treated with different peritoneal dialysis modalities.
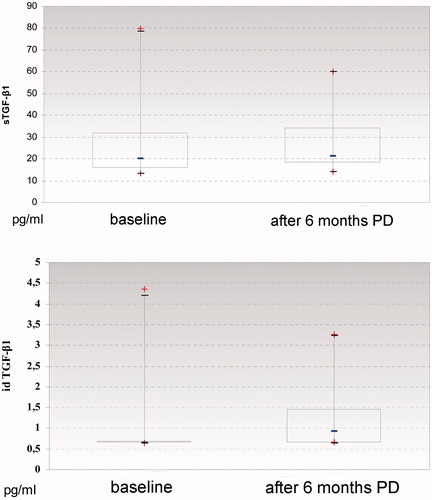
The serum TGF-β1 concentration was 26.82 ± 16.60 ng/mL (range 13.46–55.10 ng/mL) at the beginning and it was 27.96 ± 14.70 ng/mL (range 15.20–79.94 ng/mL) after 6 months of peritoneal dialysis treatment. The drained dialysate TGF-β1 concentration was 0.90 ± 0.84 ng/mL (range 0.65–4.36 ng/mL) at the beginning and it was 1.16 ± 0.73 ng/mL (range 0.65–3.27 ng/mL) after 6 months of peritoneal dialysis treatment. During the 6 months follow up the serum and drained dialysate concentrations of TGF-β1 slightly increased, with no statistically significant difference ().
Figure 1. (A) Concentrations of transforming-growth factor 1 (TGF-β1) in serum at the beginning and after 6 months of peritoneal dialysis treatment. (B) Concentrations of transforming-growth factor 1 (TGF-β1) in drained dialysate at the beginning and after 6 months of peritoneal dialysis treatment.
After 6 months of chronic peritoneal dialysis treatment concentrations of investigated biochemical parameters did not change significantly. Slight decrease was noted in serum concentrations of glucose (6.25 ± 2.38 mmol/L vs. 6.09 ±2.48 mmol/L), fibrinogen (5.50 ± 1.41 g/L vs. 5.16 ± 1.50 g/L), iron (10.77 ± 5.00 µmol/L vs. 10.30 ± 3.68 µmol/L), TIBC (38.64 ± 7.60 vs. 37.59 ± 10.92), SAT (30.50 ± 14.09% vs. 26.50 ± 7.72%), parathyroid hormone (296.60 ± 207.38 pg/mL vs. 290.50 ± 205.37 pg/mL) and total proteins (63.20 ±7.33 g/L vs. 62.85 ± 7.65 g/L), while slight rise was observed in concentrations of serum urea (18.9 ± 45.47 mmol/L vs. 18.99 ± 6.11 mmol/L), creatinine (661.25 ± 177.65 µmol/L vs. 698.85 ± 243.30 µmol/L), albumin (31.30 ± 5.20 g/L vs. 32.00 ± 5.87 g/L), CRP (6.30 ± 6.04 IU/L vs. 6.64 ± 5.63 IU/L), cholesterol (5.58 ± 1.71 mmol/L vs. 5.97 ± 2.07 mmol/L), triglycerides (1.86 ± 0.95 mmol/L vs. 2.10 ± 1.00 mmol/L), HB (99.15 ± 14.52 g/L vs. 102.95 ± 10.90 g/L), hematocrit (0.30 ± 0.04 vs. 0.31 ± 0.03) and ferritin (248.23 ±223.40 mmol/L vs. 295.09 ± 253.00 mmol/L).
At the beginning of peritoneal dialysis treatment serum TGF-β1 concentration correlated significantly with glycemia (p = 0.016); and no significant correlation was found between TGF-β1 levels in drained dialysate and any of the biochemical variables (). After 6 months of chronic PD treatment significant positive correlation was found between serum cholesterol and drained dialysate TGF-β1 levels (p < 0.0001); no significant correlation was found between TGF-β1 levels in serum and any of the biochemical variables ().
Table 1. Correlation between serum concentrations of biochemical parameters and inflammatory markers and serum and drained dialysate concentrations of TGF-β1 at the beginning and after 6 months of peritoneal dialysis treatment.
During the 6 months follow-up three patients experienced peritonitis. No significant difference was found in serum and drained dialysate TGF-β1 levels in patients free of peritonitis compared to the ones who experienced peritonitis during the 6 months follow-up ().
Table 2. Peritonitis and transforming-growth factor β1 (TGF-β1) concentrations in serum and drained dialysate after 6 months of peritoneal dialysis treatment.
In our study group only two patients did not use ACE inhibitors. No statistically significant difference was found in serum and drained dialysate TGF-β1 levels related to treatment with ACE inhibitors (). Patients on ESA therapy showed significant increase in serum TGF-β1 levels after 6 months on PD (p = 0.049), which did not reflect on drained dialysate TGF-β1 levels ().
Table 3. Influence of ESA, ACEi and statins treatment on transforming-growth factor β1 (TGF-β1) concentrations in serum and drained dialysate at baseline (0) and after 6 months (6) of peritoneal dialysis treatment.
Patients taking statins had significantly lower serum TGF-β1 concentrations (p = 0.030) and insignificantly lower drained dialysate TGF-β1 concentrations at baseline. However, after the 6 months peritoneal dialysis treatment, TGF-β1 levels were higher in patients receiving statins in both serum and drained dialysate, but the difference was not statistically significant ().
Discussion
TGF-β1 is a powerful cytokine with a major role in physiological processes of growth, differentiation and gene expression. It is essential in embryogenesis, useful in tissue remodelling, reparation and wound healing, dangerous in tumorigenesis, immunosuppression and fibrosis.Citation1 TGF-β1 enhances synthesis of extracellular matrix proteins (collagen and fibronectin) and upregulates integrins responsible for connections between cells and the extracellular matrix. It mediates the degradation of extracellular matrix proteins through complex interactions with plasmin, tissue metalloproteinases and their inhibitors.Citation1–3 Peritoneal fibrosis can be ameliorated by inhibition of TGF-β1 signaling.Citation9
In patients affected by end-stage renal failure on chronic peritoneal dialysis treatment, TGF-β1 is one of the numerous cytokines and factors upregulated and involved in peritoneal membrane deterioration. It plays a pivotal role in epithelial-to-mesenchymal transition of mesothelial cells and development of peritoneal membrane fibrosis, leading to functional alterations.Citation6,Citation7 Transfer of TGF-β1 gene by adenoviral vectors into the peritoneal cavity of experimental animals upregulates the cytokine and induces peritoneal fibrosis.Citation10
TGF-β1 is detected in cultured peritoneal mesothelial cells, where it upregulates synthesis of collagen, fibronectin and actin in α-smooth muscle cells.Citation8 High glucose content in peritoneal dialysis fluid enhances fibronectin and TGF-β1 synthesis and inhibits perlecan synthesis in cultured mesothelial cells.Citation11–14 Histological analysis of peritoneal biopsies from patients undergoing chronic peritoneal dialysis treatment shows progressive denudation of mesothelial layer and reduction of proliferation of remaining mesothelial cells, in presence of the upregulated TGF-β1 and high glucose content of dialysis fluid, compromising the regeneration of the damaged peritoneal membrane.Citation9,Citation15
The mean concentration of TGF-β1 in serum in our study group was 26.82 ± 16.60 ng/mL (13.46–79.96 ng/mL) at the beginning of peritoneal dialysis treatment and 27.94 ± 14.70 ng/mL after the 6 months follow up. Previous studies found lower concentrations of TGF-β1 in serum of patients on chronic peritoneal dialysis treatment, ranging from 1.21 to 8.62 ng/mL.Citation16
TGF-β1 can be detected in drained dialysate of patients on chronic peritoneal dialysis treatment, where it is partly transferred from systemic circulation in the peritoneal space by diffusion and partly locally synthesized in the peritoneal membrane.Citation17,Citation18 So far, there are no referent ranges defined for TGF-β concentrations in drained dialysate.Citation18 The average concentration of TGF-β1 in drained dialysate in our patients was 0.9 ± 0.84 ng/mL (0.65–4.36 ng/mL) at the beginning of chronic peritoneal dialysis treatment and it was 1.19 ± 0.79 ng/mL after the 6 months follow up. Our findings are in agreement with those by other authors, reporting the concentration of TGF-β1 locally synthesized in peritoneal membrane and detected in drained dialysate up to 293.8 ng/L,Citation18 or 22.87–474.2 ng/mL.Citation19
Previous studies reported higher TGF-β1 concentrations in serum of patients affected by end-stage renal failure during long term peritoneal dialysis treatment and in patients with peritoneal fibrosis.Citation4 Confirming these findings, the serum and drained dialysate TGF-β1 concentrations slightly rose during the first 6 months of chronic peritoneal dialysis treatment in our study group ().Citation16
Patients demographic characteristics and diabetes mellitus did not affect serum and drained dialysate TGF-β1 concentrations.
Our patients were treated with conventional glucose-based low pH peritoneal dialysis solutions, mostly with the lowest glucose concentration to avoid high glucose load. Prospective randomized cross-over as well as parallel arms studies during the past 15 years even proved no statistically significant differences in serum and drained dialysate TGF-β1 concentrations between patients treated with conventional peritoneal dialysis fluids and patients treated with new biocompatible peritoneal dialysis fluids with neutral pH and lower content of glucose degradation products.Citation20–22 Dialysis modality did not significantly influence neither the serum nor the drained dialysate TGF-β1 concentrations in our study group.
The significant positive correlations between serum TGF-β1 levels and glycemia at the beginning and between drained dialysate TGF-β1 levels and cholesterolemia after 6 months of PD treatment suggest higher TGF-β1 concentrations in patients with unfavorable metabolic profile ().
No statistically significant correlation was found in serum and drained dialysate TGF-β1 concentrations and peritonitis during the first 6 months of peritoneal dialysis treatment. Actually, peritonitis episode was even associated with slightly lower TGF-β1 levels (). We could explain our findings by the drop out of patients with clinical and laboratory signs of peritonitis and/or other systemic infection within four weeks prior to the evaluation. Literature data reported higher TGF-β1 concentrations in serum of patients experiencing peritonitis during long term peritoneal dialysis treatment.Citation4
Previous studies showed overall increase of drained dialysate TGF-β1 concentrations in patients using ACEi and/or angiotensin-receptor blockers (ARB) compared to patients not using ACEi/ARB therapy during a 12 months follow up on peritoneal dialysis treatment. Expression of TGF-β1 in effluent dialysate significantly increased in patients on chronic peritoneal dialysis not using ACEi/ARB therapy, while it only slightly increased in patients on ACEi/ARB therapy and these findings suggested ACEi/ARB to have a protective effect against peritoneal fibrosis during long-term peritoneal dialysis treatment.Citation23–25 A slight, but not statistically significant, rise was observed during the 6 months follow-up in serum and drained dialysate TGF-β1 concentrations in all patients in our study population, either receiving or not receiving ACE inhibitors ().
Patients treated with statins had statistically significant lower serum TGF-β1 concentrations at the beginning of PD treatment (), implicating a possible inhibiting effect of statins on TGF-β1 upregulation. There are no data about the effect of statins on TGF-β1 synthesis in human population on chronic peritoneal dialysis treatment. Experimental findings suggest that in mice simultaneous intraperitoneal application of losartan and atorvastatin leads to an enhanced reduction in adhesion, possibly through balancing the expression of TGF-β1.Citation26
Patients on ESA therapy had slightly lower serum and higher drained dialysate TGF-β1 concentrations after the first 6 months PD treatment (). Literature data on the influence of ESA therapy on TGF-β1 concentration in human population are missing, while it was found that the intraperitoneal application of 5000 U/kg erythropoietin three times per week during 3 weeks reduces chlorhexidine-induced experimental fibrosis in rats' peritoneum.Citation27
Conclusions
Our results suggest that at baseline serum and drained dialysate TGF-β1 concentrations were not affected neither with sex, age, diabetes mellitus, nor with different peritoneal dialysis modalities After the 6 months of peritoneal dialysis the serum and drained dialysate concentrations of TGF-β1 significantly increased and they were higher in patients with unfavorable metabolic profile identified with glucose and cholesterol levels.
In our patients starting peritoneal dialysis treatment on statins therapy TGF-β1 levels in serum and drained dialysate suggested a protective effect of statins on peritoneal membrane, but after the 6 months follow-up statins did not influence significantly TGF-β1 levels.
Treatment with ESA and ACEi, as well as peritonitis episodes did not affect significantly TGF-β1 levels in serum and drained dialysate.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor β in human disease role of transforming growth factor β in human disease. N Engl J Med. 2000;342:1350–1358
- Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S. TGFβ – A good servant but a bad master. J Transl Med. 2012;10:183. doi: 10.1186/1479-5876-10-183
- Lawrence DA. Transforming growth factor-β: A general review. Eur Cytokine New. 1996;7:363–374
- Janda K, Krzanowski M, Dumnick P, Kusnierz-Cabala A, Sulowicz W. Transforming growth factor beta 1 as a risk factor for cardiovascular disease in end-stage renal disease patients treated with peritoneal dialysis. Clin Lab. 2014;60(7):1163–1168
- Ueno T, Nakashima A, Doi S, et al. Mesenchymal stem cells ameliorate experimental peritoneal fibrosis by suppressing inflammation and inhibiting TGF-β1 signaling. Kidney Int. 2013;84(2):297–307
- McLoughlin RM, Topley N. Switching on EMT in the peritoneal membrane: Considering the evidence. Nephrol Dial Transplant. 2011;26:12–15
- Hung KY, Wu KD, Tsai TJ. In vitro study of peritoneal fibrosis. Perit Dial Int. 2007;27(Supl. 2):S72–S75
- Honda K, Kamada C, Nayama M, et al. Impact of uremia, diabetes and peritoneal dialysis itself on the pathogenesis of peritoneal sclerosis: A quantitative study of peritoneal membrane morphology. Clin J Am Soc Nephrol. 2008;3:720–728
- Margetts P, Bonniaud P, Liu L, et al. Transient overexpression of TgF-β1 induces epithelial mesenchymal transition in rodent peritoneum. J Am Soc Nephrol. 2005;16:425–436
- Garosi G. Different aspects of peritoneal damage: Fibrosis and sclerosis. In: Crepaldi C, Cruz DN, eds. Peritoneal Dialysis – From Basic Concepts to Clinical Excellence. Contributions to Nephrology. Vol. 163. Basel: Karger; 2009:45–53
- Ha H, Cha MK, Choi HN, Lee HB. Effects of peritoneal dialysis solutions on the secretion of growth factored extracellular matrix proteins by human peritoneal mesothelial cells. Perit Dial Int. 2002;22:171–177
- Randall JF. Bad and good growth factors in the peritoneal cavity. Nephrology. 2005:10:234–239
- Al-Jayyousi RH, Medcalf JF, Harris KPG. Role of transforming growth factor beta in peritoneal fibrosis. Nephrology. 2002;7:216–219
- Margetts PJ, Kolb M, Galt T, Hoff CM, Shockley TR, Gauldie J. Gene transfer of transforming growth factor beta to the rat peritoneum: Effects on membrane function. J Am Soc Nephrol. 2001;12:2029–2039
- Lin CY, Chen WP, FU LW, Huan TP. Persistent transforming growth factor β expression may predict peritoneal fibrosis in CAPD patients with frequent peritonitis occurrence. Adv Perit Dial. 1997;13:64–71
- Stompor T, Zdzienicka A, Motyka M, Dembinska-Kiec A, Davies SJ, Sulowicy W. Selected growth factors in peritoneal dialysis: Their relationship to markers of inflammation, dialysis adequacy, residual renal function and peritoneal membrane transport. Perit Dial Int. 2002;22:670–676
- Zweers MM, deWaart DR, Smit W, Struijk DG, Krediet RT. Growth factors VEGF and TGF in peritoneal dialysis. J Lab Clin Med. 1999;134:124–132
- Devuyst O. Molecular mechanisms of peritoneal permeability – Research in growth factors. Perit Dial Int. 2001;21:S19–S23
- Jones S, Clifford JH, Krediet RT, et al., on behalf of the Bicarbonate/Lactate Study Group. Bicarbonate/lactate-based peritoneal dialysis solution increases cancer antigen 125 and decreases hyaluronic acid levels. Kidney Int. 2001;59:1529–1538
- Fusshoeller A, Plail M, Grabensee B, Plum J. Biocompatibility pattern of a bicarbonate/lactate-buffered peritoneal dialysis fluid in APD: A prospective randomized study. Nephrol Dial Transplant. 2004;19:2101–2106
- Theodoridis M, Thodis B, Tsigalou C, et al. Alterations of dialysate markers in chronic peritoneal dialysis patients treated with the new less biocompatible solutions. Perit Dial Int. 2011;31:196–199
- Weiss L, Stegmayr B, Malmsten G, et al. Biocompatibility and tolerability of a purely bicarbonate-buffered peritoneal dialysis solution. Perit Dial Int. 2009;29:647–655
- Jing S, Kezhou Y, Hong Z, Qun W, Rong W. Effects of renin–angiotensin system inhibitors on prevention of peritoneal fibrosis in peritoneal dialysis patients. Nephrology (Carlton). 2010;15(1):27–32
- Lee CJ, Subeq YM, Lee RP, Liou HH, Hsu BG. Calcitriol decreases TGF-β1 and angiotensin II production and protects against chlorhexidine digluconate-induced liver peritoneal fibrosis in rats. Cytokine. 2014;65(1):105–118
- Duman S, Wieczorowska-Tobis K, Styszynski A, Kwiatkowska B, Breborowicz A, Oroepoulos DG. Intraperitoneal enalapril ameliorates morphologic changes induced by hypertonic peritoneal dialysis solutions in rat peritoneum. Adv Perit Dial. 2004;20:31–36
- Dinarvand P, Farhadian S, Seyedjafari E, et al. Novel approach to reduce postsurgical adhesions to a minimum: Administration of losartan plus atorvastatin intraperitoneally. J Surg Res. 2013;181(1):91–98
- Mondello S, Mazzon E, Di Paola R, et al. Erythropoietin suppresses peritoneal fibrosis in rat experimental model. Eur J Pharmacol. 2009;604:138–149
