Abstract
Heparin is routinely administered at high doses during hemodialysis to patients with hypercoagulable states. This study aimed to evaluate the safety and efficacy of low-dose heparin in combination with urokinase in this patient population. The presence of a hypercoagulable state was confirmed by thromboelastography. Doses of heparin and urokinase were adjusted based on activated partial thromboplastin time (APTT). Clotting in the extracorporeal circuit was evaluated by a semi-quantitative index. Prothrombin time (PT) and APTT were measured before, during and after dialysis. Kt/Vurea was used to assess the efficacy of dialysis. D-dimer levels were measured 2 h after the start of hemodialysis. Hemodialysis data with heparin administered alone prior to dialysis were used as control in the present study. With urokinase treatment, the initial dose of heparin was reduced by 45.0 ± 11.4% during hemodialysis and the maintenance dose by 46.8 ± 12.8% compared with heparin alone. No side effects due to urokinase were observed. Bleeding events were rare. Post-dialysis PT (12.99 ± 1.41 vs. 15.22 ± 3.12 s, p = 0.02) and APTT (97.75 ± 43.62 vs. 140.16 ± 30.12 s, p = 0.002) with urokinase plus heparin were significantly shorter than with heparin alone. Finally, during dialysis, D-dimer levels were significantly higher with heparin alone (0.21 ± 0.11 mg/L) than with heparin and urokinase (0.169 ± 0.122 mg/L, p = 0.017). In conclusion, urokinase significantly reduced the dose of heparin required during hemodialysis without any side effects in patients with hypercoagulable states during hemodialysis.
Introduction
The goal of anticoagulation during hemodialysis is to avoid clotting, and more importantly, to optimize dialysis and to facilitate dialyzer reuse. Indeed, there is a need to reuse dialyzers in order to reduce the costs of hemodialysis because of the perceived need to use more expensive instruments to improve the efficiency of dialysis and patient survival.Citation1 Despite these efforts, some patients experience clotting irrespective of the dose of heparin administered. In these patients, routinely attained reuse rates cannot be achieved even with high-dose heparin, because of a hypercoagulable state during the procedure. Such patients require a protocol of anticoagulation with high-dose heparin, which carries an increased risk of hemorrhage, dyslipidemia, thrombocytopenia, osteoporosis or induced heparin resistance.Citation2,Citation3 In addition, patients with a hypercoagulable state during hemodialysis often experience dysfunction of tunneled cuffed catheters (TCCs) in deep veins. Endoluminal tissue plasminogen activator (t-PA) or urokinase is recommended for thrombolysis in cases where blood cannot be aspirated from the lumen or in case of inadequate blood flow.Citation4,Citation5 It has been shown that high-dose heparin, administered alone, is not sufficient to prevent clotting during hemodialysis or achieve ideal dialyzer reuse rates in these patients, even if activated partial thromboplastin time (APTT) is prolonged beyond the appropriate range (i.e., >150 s) in the absence of bleeding complications. High doses of low-molecular-weight heparin prolong the period of risk of hemorrhage because of its extended half-life and its effects cannot be completely neutralized by protamine.Citation6,Citation7 Heparin combined with oral anticoagulants such as aspirin has the potential to increase the risk of hemorrhage of the gastrointestinal mucosa. Other methods have included the use of warfarin combined with heparin in an attempt to obtain an international normalized ratio between 2 and 3.Citation5,Citation8,Citation9 Saline flushes during hemodialysis are unable to reduce clotting when combined with reduced doses of heparin in patients with stable hemodialysis.Citation10 In addition, the use of citrate is limited by the need for additional equipment, the potential risk of electrolytic and acid–base disequilibrium and the need for clotting time evaluations. The use of citrate for anticoagulation during hemodialysis can alter parathyroid hormone secretion.Citation11,Citation12 Prostacyclin is costly and requires close hemodynamic monitoring.Citation13 Argatroban is also costly;Citation14 it is mainly reserved for patients with heparin-induced thrombocytopenia and does not reduce the risk of hemorrhage.Citation15 By contrast, urokinase has been shown to prevent blood clot formation during hemodialysis by thrombolysis, and heparin used in combination with urokinase further decrease platelet aggregation. Thus, these two treatments can be considered to assist anticoagulation in two different ways: urokinase decreases the size of clots and heparin reduces their growth. In addition, urokinase has a short half-life, which reduces the risk of bleeding after dialysis.
In the present study, we evaluated the safety and efficacy of low-dose heparin in combination with urokinase in patients with hypercoagulable states during hemodialysis.
Materials and methods
Study design
This was a prospective, comparative study of combined dialysis therapy with heparin and urokinase versus pretrial maintenance dialysis therapy with heparin, in which patients served as their own controls.
For inclusion into the study the patients were required to have a functioning fistula and a dialyzer reuse rate less than the average reuse rate for the center. Patients were excluded if they had infections, chronic liver disease, cirrhosis or disseminated intravascular coagulopathy. Patients with amyloidosis, eosinophilia, fever or acute myocardial infarction were also excluded from the study as were those with active cancer or thrombophlebitis or evidence of empyrosis or hypotension during hemodialysis. None of the patients were receiving oral anticoagulants, nitroglycerin, contraceptive agents, aprotinin, digitalis or antihistamine.
The 18 cases assessed herein were patients with hypercoagulable states who underwent regular hemodialysis in our center for more than 3 months between March 1 and October 1, 2008. During this period, a total of 168 cases of chronic renal failure were observed, with an average number of dialyzer reuses of 9.5; however, this number was only 5.5 in the patients with hypercoagulable states evaluated herein. Of note, the 18 patients with hypercoagulable states showed no significant differences in age, primary disease and dialysis related data, compared with the other patients.
The study protocol was approved by the Ethics Committee of the Jinan City Central Hospital, China. All patients provided informed consent prior to inclusion in the study.
Dialysis prescription and procedures
Heparin is commonly administered as a low-dose bolus injection (25–30 U/kg) before the initiation of dialysis, followed by continuous high-dosage infusion (1500–2000 U/h) during dialysis.Citation2 An alternative regimen is to administer an initial high dose (50 U/kg) of heparin followed by continuous low-dosage infusion (1000–1500 U/h).Citation16 At our institution, we routinely use a high initial dose of 92 U/kg (range 67–150 U/kg). In the present study, all patients received a modified anticoagulation protocol comprising reduced dose of heparin with urokinase.
The dialysis prescriptions for individual patients remained unchanged during the study period. All patients underwent dialysis three times a week with volume-controlled dialysis delivery systems (4008S and 4008E, Fresenius Medical Care, Bad Homburg vor der Höhe, Germany). Ultrafiltration rates were set according to each patient's clinical need. Standard dialysis solutions were used. Polyamix™ (Polyflux 6LR; Gambro Dialysatoren GmbH, Hechingen, Germany) or cellulose diacetate (CAHP130, CAHP150; Baxter Healthcare Corp, Japan) dialyzer membranes were used for all patients. The type of dialyzer remained constant throughout the study.
Two needles were used for vascular access. Blood flow was maintained between 200 and 320 mL/min, and did not change in individual subjects during the observation period. The dialysate flow was 500 mL/min. None of the patients received additional infusions during hemodialysis.
Blood sampling
At each dialysis session, blood samples were taken from the arterial line before dialysis, after 2 h and immediately at the end of the procedure. Thromboelastography (TEG; TEG5000, Hemoscope, Niles, IL) was used to measure pre-dialysis parameters. Hemostatic parameters included PT, APTT, fibrinogen and D-dimer levels. Additional measurements were made for platelets, hemoglobin, blood urea nitrogen (BUN) and creatinine. Routine assay methods were used for all tests.
Dialysis efficiency
The dose of dialysis was determined weekly as Kt/Vurea according to the method described by Daugirdas.Citation17 The efficacy of hemodialysis was assessed as:
where ln is the natural logarithm; R is the ratio of post-dialysis BUN to pre-dialysis BUN; UF is ultrafiltration; W is post-dialysis body weight.
The urea reduction rate (URR) was estimated at 1 − R.
Clinical observation of coagulation
Clotting in the dialyzer, arterial and venous bubble catchers, examined visually, were evaluated using a 4-point coagulation index (CI) where: 0 = no detectable clotting; 1 = minimal clotting; 2 = moderate clotting; 3 = major clotting but dialysis still possible and 4 = complete occlusion of dialyzer or bubble catcher.Citation18 The duration of manual pressure on the shunt puncture sites (SPSs) post-dialysis was recorded. Hemostasis time was recorded after a 5-min compression bandage. After this 5 min, the bandage was taken off and manual compression was pursued. Hemostasis was assessed every minute until bleeding had stopped.
Dialyzer reprocessing
All dialyzers were processed for reuse using an automated Renatron system (Minntech Corp, Minneapolis, MN) with Renalin (Minntech) utilized as germicide. Dialyzer was rejected for further use in case of clotting during dialysis, or if the dialyzer failed volume or pressure tests, had blood leaks, was aesthetically unacceptable, or had reached its maximum number of uses.
Anticoagulant
At our institution, the routine concentration of unfractionated heparin is 1250 U/mL (i.e., 12,500 U heparin in 8 mL saline). The dose of heparin was adjusted according to APTT, the most common means for monitoring its anticoagulant effect.Citation19,Citation20 A 1.5- to 3.5-time increase in APTT compared with baseline values is generally thought to provide adequate anticoagulation with unfractionated heparin.Citation20 However, clots may occur during hemodialysis in the absence of bleeding complications even if the APTT is increased beyond this range (i.e., with APTT > 150 s). Thus, the dose of heparin was adjusted accordingly to maintain the coagulation index at 3 or less in the dialyzer and air traps.
Unfractionated heparin (6250–12,500 U) and urokinase (10–20 × 104 U in 6–10 mL) were mixed to facilitate administration. The concentrations of heparin and urokinase were adjusted according to thromboelastography (TEG) and APTT results pre-dialysis, and the dose was tuned according to the degree of clotting in the dialyzer and air traps.
Costs
The monthly costs of hemodialysis with and without urokinase were compared.
Statistical analysis
Values are presented as median and ranges or means and standard deviations (±SD), as appropriate. Hemostatic parameters, platelets and hemoglobin before and after combined treatment were compared using paired Student's t-tests. A similar procedure was used to compare the extent of coagulation in the air traps and dialyzer during each observation period. p-Values < 0.05 were considered statistically significant. Statistical analysis was performed using SPSS 18.0 (IBM, Armonk, NY).
Results
The study included 18 patients (15 men and 3 women) with a mean weight of 59.9 ± 9.4 kg and a mean age of 63.0 ± 15.4 years. The patients had been receiving chronic hemodialysis for 5 years in average (range 1.7–11.7 years). The cause of end-stage renal disease was glomerulonephritis (n = 8), diabetes mellitus (n = 4), hypertension (n = 2), nephrotic syndrome (n = 2), pyelonephritis (n = 1) and gouty kidney (n = 1). All patients had dialysis catheter dysfunction, with thrombosis in the catheter. In addition, some of them had AV access blocking, excluding other reasons such as compression or vascular factors. The patients' baseline characteristics are shown in .
Table 1. Patient characteristics.
Thromboelastography pre-dialysis revealed the presence of hyperactivated platelets with a maximum amplitude (MA) > 69 mm in 12 patients and hyperactivated platelets with blood coagulation factors (CI > 3) were found in six patients: clotting time (R) was < 2 min and MA was >69 mm. Pre-dialysis values for platelets, hemoglobin, fibrinogen, prothrombin time (PT), APTT and D-dimer did not differ between urokinase and control treatment ().
Table 2. Serologic parameters for patients receiving heparin during dialysis and with urokinase treatment.
The initial dose of heparin before dialysis (control) was 92.84 ± 20.28 U/kg, and the maintenance dose was 34.64 ± 5.32 U/kg. The initial dose of heparin in combination with urokinase was 49.32 ± 10.71 U/kg (p < 0.0001 vs. heparin alone) and the maintenance dose was 18.12 ± 4.17 U/kg (p < 0.0001 vs. heparin alone). The total dose of urokinase during hemodialysis was 11.53 ± 3.75 × 104 U. With urokinase treatment, the initial dose of heparin was reduced by 45.0 ± 11.4% during hemodialysis and the maintenance dose by 46.8 ± 12.8% compared with control treatment (heparin alone).
Post-dialysis PT (12.99 ± 1.41 vs. 15.22 ± 3.12 s, p = 0.02) and APTT (97.75 ± 43.62 vs. 140.16 ± 30.12 s; p = 0.002) with urokinase plus heparin were significantly shorter than with heparin alone (). During dialysis with urokinase, APTT increased by 137.9 ± 23.89 s (4.5 ± 0.90 times above baseline) and beyond the range (more than 150 s) with the control treatment.
Figure 1. Prothrombin time (PT), activated partial thromboplastin time (APTT) and D-dimer values pre- and post-dialysis with or without urokinase treatment. (a) PT, (b) APTT and (c) D-dimer pre-dialysis in patients with urokinase was not significantly different from heparin only controls. However, post-dialysis PT and APTT in urokinase treated patients were significantly shorter than in those treated with heparin only (*p < 0.05). D-dimer was markedly higher during dialysis in patients with heparin only than combination treated individuals (*p < 0.05).
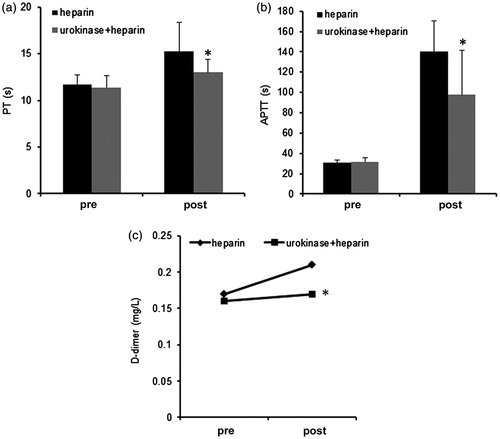
At the end of dialysis with urokinase and heparin, APTT increased by 3.09 ± 1.38-times in comparison with a 4.6 ± 1.1-time increase observed with heparin alone (p = 0.0005).
During dialysis, D-dimer levels were significantly higher with heparin alone (0.21 ± 0.11 mg/L) than with heparin and urokinase (0.169 ± 0.122 mg/L; p = 0.017). Post-dialysis fibrinogen levels after heparin and urokinase treatment (3.78 ± 0.77 g/L) did not significantly differ from those obtained with heparin alone (3.94 ± 0.59 g/L; p = 0.10).
The degree of coagulation within the dialyzers during dialysis was significantly reduced, from a mean CI of 2.66 ± 0.73 obtained with heparin to 1.23 ± 0.43 after heparin plus urokinase treatment (p < 0.05). In addition, the reuse rate increased, from 5.5 ± 2.36 to 9.4 ± 0.96 with urokinase treatment (the maximum number of uses was 10).
The extent of thrombosis in the arterial air traps was similar between urokinase plus heparin (1.11 ± 0.23) and heparin (1.10 ± 0.32; p = 0.34). The same was true for the extent of thrombosis in the venous air traps (1.08 ± 0.22 vs. 1.06 ± 0.21; p = 0.33).
The time of manual pressure on the SPSs was significantly shorter with urokinase treatment (19.64 ± 5.36 min) than with heparin alone (73.57 ± 48.8 min; p = 0.018).
Control treatment with heparin alone and combined treatment with urokinase produced similar weekly Kt/Vurea values (3.80 ± 0.19 vs. 3.81 ± 0.21, p = 0.43) and URR values (0.72 ± 0.02 vs. 0.73 ± 0.03, p = 0.44). No side effects were observed with urokinase during or after dialysis.
The monthly cost per patient was reduced by approximately 324.32 ± 30.01¥ (from 1298.57¥ to 974.25¥) after treatment with urokinase in comparison with heparin alone.
Discussion
In this study, we evaluated the safety and efficacy of low-dose heparin in combination with urokinase on extracorporeal circuit clotting in patients with hypercoagulable states during hemodialysis. We demonstrated that the use of heparin combined with urokinase resulted in a significant increase in reuse rate, which was independent of heparin dose. In fact, the total dose of heparin was approximately 46.3% lower than that usually administered. Thus, the use of urokinase in patients with a hypercoagulable state during dialysis significantly reduced the dose of heparin required during hemodialysis to prevent extracorporeal circuit clotting without any side effects.
The use of urokinase has been shown to transform plasminogen to plasmin, a strong serine proteinase involved in the degradation of fibrinogen to fragments X, Y, D, E and Bβ1-42. As a result of the reduction in size of blood clots, the blood changes from hyper- to hypo-coagulation state by the formation of fragments X(X′), Y(Y′), D, E(E′). This in turn inhibits platelet aggregation and coagulation, allowing the dose of heparin to be reduced and the associated risk of side effects to be minimized.
Dialyzer clotting is a complex phenomenon involving activation of the coagulation cascade and platelets by the non-physiological materials and fluid dynamics of the extracorporeal circuit.Citation21 Therefore, this non-physiological environment will increase the risk of clotting. In addition, factors from the patients themselves will increase the risk of extracorporeal clotting. The thrombophilia of uremic platelets is attributed in part to the translocation of phosphatidylserine to the outer membrane due to chronic platelet activation in patients on chronic hemodialysisCitation22 and treatment with erythropoietin.Citation23 Erythropoietin not only improves platelet function by correcting anemia but also has direct effects of increased number of GPIIb/IIIa receptors on platelets. It enhances thrombin-induced phosphorylation of platelet proteins and increases microparticles with procoagulant activity.Citation23 In this study, pre-dialysis TEG revealed that all patients had hyper-activated platelets, and about 30% had hyper-activated blood coagulation factors. These findings suggest that hyper-activated platelets may be the main cause of the hypercoagulable disorders leading to clotting.
D-dimers are products of fibrin hydrolysis and are usually found in patients with suspected deep-venous thrombosis or pulmonary emboli; however, they can also be used as a general marker for thrombus formation. D-dimer serum concentration is closely associated with thrombin–antithrombin complex formation, and thus coagulation, during hemodialysis.Citation17,Citation24 Our results identified the sensitivity of D-dimer as a marker for coagulation activity as we demonstrated a significant association between clotting in the dialyzer and increased D-dimer levels. Monitoring D-dimer levels might, therefore, be useful for estimating the extent of coagulation during hemodialysis. The lack of efficacy of heparin alone in preventing coagulation was demonstrated by increased D-dimer plasma levels and enhanced clotting in the dialyzer in comparison with combined treatment with heparin and urokinase.
The dialyzers were discarded if they failed the test for post-dialysis blood compartment volume performed by the Renatron (i.e., if the measured volume was 80% or less the measured volume of the dialyzer before its first use). They were also discarded if the maximum number of uses recommended by the manufacturer was reached. Thus, the dialyzers were changed when thrombosis within the equipment influenced the dialysis dose to some extent. This meant that overall dialysis dose indices such as Kt/Vurea and URR remained fairly constant before and after changing the anticoagulant protocol.
Although Kt/Vurea and URR were slightly lower with heparin alone than with heparin and urokinase, the difference was not significant. This was probably due to the lack of statistical power attributable to the small sample size and the short observation period. It may also have been the result of changing to a different type of dialyzer from the one used prior to the study.
One complication of anticoagulation is the risk of bleeding. A meta-analysis comparing the safety of low-molecular-weight heparin and unfractionated heparin (UFH) during dialysis showed that “major” bleeding events with either therapy were so rare that this outcome had to be grouped with “minor” bleeding to facilitate an overall statistical analysis.Citation4 Access-related bleeding was the most common event in the individual studies cited in this meta-analysis.
None of the patients in our study experienced overt complications that could be attributed to bleeding. However, post-dialysis PT, SPSs and APTT were significantly lower with urokinase treatment than with the control treatment. The half-life of urokinase is 20 min, and anticoagulant infusions were stopped approximately 30 min prior to the end of dialysis. Thus, the main anticoagulant remaining at the end of the procedure was heparin. Nevertheless, patients treated with urokinase had a lower risk of access-related bleeding than those receiving heparin alone.
Despite the cost of urokinase, the monthly costs of dialysis in patients who received urokinase and heparin were lower than for patients receiving heparin alone, mainly because of the higher reuse of the dialyzer units. shows the costs when exchanging to US dollars, euro or Japanese Yen.
Table 3. Costs (per month) of the heparin alone versus heparin + urokinase approaches in patients with hypercoagulable states under dialysis.
Further studies are required to investigate whether a reduced dose of heparin in combination with urokinase is able to reduce other complications associated with administration of large-dose unfractionated heparin in patients with dyslipidemia, thrombocytopenia and osteoporosis. For instance, large-dose-induced severe heparin resistance was identified as an independent predictor of death in coronary patients, suggesting that increased attention should be paid to this phenomenon.Citation25 In addition, the sample size was small, which was due to the fact that they were from a single center, but also because of the strict inclusion/exclusion criteria we used to minimize variability between patients. Nevertheless, a multicenter study should be conducted.
In conclusion, our results demonstrate that the use of urokinase in patients with hypercoagulable states during hemodialysis enables a reduced dose of heparin to be used without increasing the incidence of side effects. The reduced dose of heparin in combination with urokinase appears to decrease the risk of bleeding after dialysis. The cost of hemodialysis is also reduced.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ouseph R, Brier ME, Ward RA. Improved dialyzer reuse after use of a population pharmacodynamic model to determine heparin doses. Am J Kidney Dis. 2000;35(1):89–94
- Sonawane S, Kasbekar N, Berns JS. The safety of heparins in end-stage renal disease. Sem Dial. 2006;19(4):305–310
- Bharadwaj J, Jayaraman C, Shrivastava R. Heparin resistance. Lab Hematol. 2003;9(3):125–131
- Lo DS, Rabbat CG, Clase CM. Thromboembolism and anticoagulant management in hemodialysis patients: A practical guide to clinical management. Thromb Res. 2006;118(3):385–395
- Webb A, Abdalla M, Russell GI. A protocol of urokinase infusion and warfarin for the management of the thrombosed hemodialysis catheter. Nephrol Dial Transplant. 2001;16(10):2075–2078
- Guillet B, Simon N, Sampol JJ, et al. Pharmacokinetics of the low molecular weight heparin enoxaparin during 48 h after bolus administration as an anticoagulant in hemodialysis. Nephrol Dial Transplant. 2003;18(11):2348–2353
- Farooq V, Hegarty J, Chandrasekar T, et al. Serious adverse incidents with the usage of low molecular weight heparins in patients with chronic kidney disease. Am J Kidney Dis. 2004;43(3):531–537
- Schetz M. Anticoagulation for continuous renal replacement therapy. Curr Opin Anaesthesiol. 2001;14(2):143–149
- Davenport A. Anticoagulation options for intermittent hemodialysis. Minerva Urol Nefrol. 2006;58(2):171–180
- Sagedal S, Hartmann A, Osnes K, et al. Intermittent saline flushes during hemodialysis do not alleviate coagulation and clot formation in stable patients receiving reduced doses of dalteparin. Nephrol Dial Transplant. 2006;21(2):444–449
- De Vos J, Hombrouckx R. Citrate anticoagulation and adverse events. EDTNA/ERCA J. 2003;29(3):112–113, 117
- Apsner R, Gruber D, Horl WH, Sunder-Plassmann G. Parathyroid hormone secretion during citrate anticoagulated hemodialysis in acutely ill maintenance hemodialysis patients. Anesth Analg. 2004;99(4):1199–1204, table of contents
- Balik M, Waldauf P, Plasil P, Pachl J. Prostacyclin versus citrate in continuous hemodiafiltration: An observational study in patients with high risk of bleeding. Blood Purif. 2005;23(4):325–329
- Ferguson LM, Dreisbach AW, Csongradi E, Juncos LA, Fulop T. Recurring extracorporeal circuit clotting during continuous renal replacement therapy in fungal sepsis: Successful treatment with argatroban. Am J Med Sci. 2013;345(3):256–258
- Tang IY, Cox DS, Patel K, et al. Argatroban and renal replacement therapy in patients with heparin-induced thrombocytopenia. Ann Pharmacother. 2005;39(2):231–236
- Ward RA. Heparinization for routine hemodialysis. Adv Renal Replace Ther. 1995;2(4):362–370
- Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J Am Soc Nephrol. 1993;4(5):1205–1213
- Ziai F, Benesch T, Kodras K, Neumann I, Dimopoulos-Xicki L, Haas M. The effect of oral anticoagulation on clotting during hemodialysis. Kidney Int. 2005;68(2):862–866
- Reiner JS, Coyne KS, Lundergan CF, C B. Superiority of bedside partial thromboplastin time to activated clotting time in controlling heparin anticoagulation. J Am Coll Cardiol. 1993;21:773–771
- Eiswirth G, Walch S, Bommer J. New bedside test for monitoring anticoagulation during hemodialysis. Artif Organs. 1998;22(4):346–348
- Ward RA. Effects of hemodialysis on coagulation and platelets: Are we measuring membrane biocompatibility? Nephrol Dial Transplant. 1995;10(Suppl 10):12–17
- Bonomini M, Dottori S, Amoroso L, Arduini A, Sirolli V. Increased platelet phosphatidylserine exposure and caspase activation in chronic uremia. J Thromb Hemost. 2004;2(8):1275–1281
- Kaw D, Malhotra D. Platelet dysfunction and end-stage renal disease. Semin Dialysis. 2006;19(4):317–322
- Hafner G, Swars H, Ehrenthal W, Schinzel H, Weilemann LS, Prellwitz W. The control of anti-coagulation in acute dialyses with sensitive laboratory parameters. Scand J Clin Lab Invest. 1992;52(4):289–296
- Knapik P, Ciesla D, Przybylski R, Knapik T. The influence of heparin resistance on postoperative complications in patients undergoing coronary surgery. Med Sci Monit. 2012;18(2):CR105–111
