Abstract
The aim of this study was to develop a population pharmacokinetic (PK) model for clearance of mycophenolic acid (MPA) in adult renal transplant recipients, to quantify the PK parameters and the influence of covariates on the MPA pharmacokinetic parameters. Parameters associated with plasma concentrations of MPA at steady-state were analyzed in 70 renal transplant recipients (mean age 42.97 years; mean total body weight 75.33 kg) using nonlinear mixed-effect modeling (NONMEM). Characteristics of patients screened for influence on the pharmacokinetic parameters were gender, age, body weight, time after transplantation, whether the patient was diagnosed as having diabetes mellitus, organ source (living or deceased donor), biochemical parameters and co-therapy (tacrolimus, cyclosporine, prednisolone, omeprazole, bisoprolol, carvedilol, nifedipine). A validation set of 25 renal transplant recipients was used to estimate the predictive performance of population pharmacokinetic model. Typical mean value of MPA oral clearance, estimated by base model (without covariates) was 0.741 L h−1. During population modeling, the full model showed that clearance of the MPA was significantly influenced by age, total daily dose of MPA, creatinine clearance, albumin level, status and gender of a donor, and the nifedipine and tacrolimus co-therapy. In the final model, clearance of MPA was reported to be significantly influenced by age, total daily dose of MPA and thenifedipine co-therapy. The derived model describes adequately MPA clearance in terms of characteristics of our patients, offering basis for individual pharmacotherapy approach.
Introduction
Mycophenolate mofetil (MMF) is the most widely used antiproliferative immunosuppressive drug in patients after solid organ transplantation, often administered in combination with calcineurin inhibitors (CNIs) and corticosteroids.Citation1 Clinical trials in renal transplant patients have demonstrated that MMF is more efficacious as an immunosuppressant than azathioprine or placebo.Citation2–4 Following oral administration, MMF is completely absorbed and rapidly hydrolyzed to the active metabolite – mycophenolic acid (MPA).Citation5 The MPA is a selective, uncompetitive and reversible inosine monophosphate dehydrogenase inhibitor, which has an important role in guanosine triphosphates de novo synthesis. Consequently, MPA inhibits the proliferation of T and B lymphocytes, preventing the acute rejection of a transplanted organ as well as decreasing the frequency of late rejection.Citation6,Citation7 MPA is mainly metabolized into the pharmacologically inactive MPA glucuronide (MPAG) by hepatic and renal glucuronosyl transferases (UGT).Citation8 Due to enterohepatic recirculation MPAG plays an important role in the maintenance of steady-state plasma MPA level.Citation8,Citation9
Most studies showed little correlation between MPA pharmacokinetic parameters and its adverse effects.Citation10–12 Gastrointestinal adverse events are frequently observed in renal transplant recipients treated with MMF. To avoid these gastrointestinal adverse events and improve the clinical outcome, enteric-coated mycophenolate sodium (EC-MPS) was developed.Citation13 At a dose of 720 mg, EC-MPS showed similar efficacy and safety profiles as 1000 mg of MMF.Citation14–16 The pharmacokinetics (PK) of MPA in renal transplant recipients is characterized by a large inter- and intra-individual variability, particularly in the early post-transplant period.Citation17,Citation18 A number of factors have been reported to alter the pharmacokinetics of MPA, including concomitant immunosuppressant administration (tacrolimus or cyclosporine A), food intake, analytical technique for MPA measurement, time after transplant and pharmacogenetic factors.Citation8,Citation19–21 The MPA pharmacokinetics will be especially variable in the early post-transplant period due to delayed graft function, hypoalbuminemia, hyperbilirubinemia, altered gastrointestinal motility and widely varying drug absorption. Hypoalbuminemia is probably the most important factor influencing MPA free fraction because 97% of MPA binds to plasma proteins (albumin) in patients with normal renal and liver function. Some changes in free MPA concentrations, due to patient characteristics such as severe renal impairment and gender, will lead to an altered efficacy or toxicity profile.Citation5,Citation8,Citation22,Citation23
The aim of this study was to develop a population pharmacokinetic model for MPA in adult renal transplant recipients, to quantify the population pharmacokinetic parameters, the variability between and within patients and the influence of covariates on the MPA pharmacokinetic parameters.
Materials and methods
Patients
Population pharmacokinetic analysis was performed in 95 Serbian renal transplant recipients. The patients were treated in the Clinic of Nephrology (University Clinical Centre of Niš, Serbia) during 2014. Patients gave written informed consent and all investigations were approved by the Ethical committee (Faculty of Medicine, University of Niš). For the purpose of this study, blood samples were obtained from the patients after steady-state was reached.
Patients received oral MMF (Cellcept®, Roche, Basel, Switzerland) or EC-MPS (Myfortic®, Novartis Pharma, Basel, Switzerland) as part of a “triple” immunosuppressive regimen, which also included corticosteroids (prednisolone) and cyclosporine or tacrolimus. The dose of EC-MPS and MMF varied from 0.54 to 1.44 g/day and from 0.50 to 2.00 g/day, respectively. It was administered in two divided doses, 12-hourly. To be able to compare measured MPA blood levels in both group, doses of MMF and EC-MPS were converted to the equivalent MPA content by multiplying the MMF dose with 0.72.Citation14–16
Inclusion criteria were patients who received transplants and were followed in our center, patients with stable graft function and immunosuppression regimen of tacrolimus or cyclosporine A-based therapy for at least three months prior to enrollment. Exclusion criteria included patients with acute rejection episodes, with a shift to other immunosuppressive regimen during the follow-up period and creatinine clearance <15 mL/min. Before analysis, the 95 patients were randomly allocated to either the model building group (n = 70) or the validation group (n = 25).
Methods
Biochemical parameters such as serum creatinine (sCr), creatinine clearance (CLCR), serum albumin, leukocytes (le), urea, hemoglobin, aspartate amino transferase (AST), alanine amino transferase (ALT) were monitored on the study date. CLCR was calculated from serum creatinine values using the standard Cockcroft and Gault equation.Citation24
Characteristics of patients screened for influence on the pharmacokinetic parameters were gender, age, body weight, time after transplantation, whether the patient was diagnosed as having diabetes mellitus, organ source (living or deceased donor), biochemical parameters and co-therapy (tacrolimus, cyclosporine A, prednisolone, omeprazole, bisoprolol, carvedilol, nifedipine).
Whole blood samples were collected into vacuum tubes containing ethylene diamine tetraacetic acid (EDTA) as an anticoagulant. Plasma was removed after centrifugation (15 min, 3000 r/min) and stored at −20 °C until analysis. High-performance liquid chromatography (HPLC) method combined with protein precipitation has been developed and validated for the analysis of MPA in human plasma.
Sample preparation
A 200 µL aliquot of patient plasma was transferred into a 1.0 mL Eppendorf tube followed by 10 µL of 1 mg/mL propylparaben (internal standard) in acetonitrile and 490 µL of 0.3% trifluoroacetic acid in acetonitrile (v/v).
Each tube was capped, vortex mixed for 1 min, and subsequently centrifuged for 10 min at 10,000 rpm and temperature of 4 °C. Supernatant was transferred into a clean auto-sampler vial and 10 µL was injected into HPLC system for analysis.
HPLC analysis
HPLC analysis was performed using a C18 Bakerbond-BDC analytical column (250 mm × 4.6 mm, particle size 5 µm). The optimal conditions for the separation were established with the mobile phase acetonitrile–10 mM phosphate buffer pH 2.5 (50:50, v/v) at a flow rate of 1.0 mL/min and temperature of 30 °C, with UV detection at 215 nm. Chromatographic run time was about 6 min. HPLC method combined with protein precipitation was subjected to validation. Limit of detection and limit of quantification were 0.025 and 0.2 μg/mL, respectively, for MPA. Linearity was obtained over the concentration range of 0.2–100 μg/mL for MPA with correlation coefficient equal to 0.9995. The method showed good intra-day and inter-day precision with a relative standard deviation below 8.08%, while the accuracy ranged from 89.31% to 102.53% for MPA.
Pharmacokinetic analysis
The NONMEM software with ADVAN4 TRANS3 subroutine (version 6, level 1.1) and G77 FORTRAN compiler were used to estimate the mean population value of MPA clearance and determine the influence of different covariate.Citation25 It is two-compartment model with lag-time and first-order absorption. As all doses were given orally, absolute bioavailability (F) of the drug were not assessed.
Software analysis of collected data with initial values of parameters from the literature gave us estimates of apparent oral clearance (CL/F), apparent volume of distribution (Vd/F), apparent volume of distribution in steady-state (VSS/F), intercompartment clearance (Q/F), absorption rate constant (Ka), lag time between intake and absorption start (lag time) and interindividual and residual variability. Also, one of the requirements in the current phase is estimation of the most appropriate error models (additive or exponential). It is the base pharmacokinetic model without covariates. In addition, further analysis included examination of influence of all covariates on MPA clearance (body weight, age, gender, total MPA dose, creatinine clearance, plasma albumin level, time after transplantation, donor type, gender of donor, AST, ALT, co-medication with omeprazole and nifedipine, dose of cyclosporine, tacrolimus and prednisolone). It was carried out by adding of every single covariate to the base model using linear or nonlinear regression with assesses of statistical significance. Value of minimum objective function (MOF) is primary statistical parameter defined as 2loglikelihood (−2LL), so for each covariate the MOF reduction was necessary of at least 3.841 (χ2 = 3.841 for p < 0.05, df = 1).Citation26 Several univariate regression models have been obtained but only models and covariate with a significant reduction in the MOF values were important for further analysis. The full model was the result of this process and only covariates that satisfied statistical requirements were included simultaneously. For deriving the final pharmacokinetic model, a process of backward deletion of covariates with more restrictive statistical criterion (increase in the MOF of more than 6.6 for p < 0.01, df = 1) was performed. Also, during all phases of population pharmacokinetic (PPK) analysis, was necessary to satisfy the following requirements: reduction in interindividual and residual variability, improvement of scatter-plots of predicted values (PRED) versus observed concentrations (DV) and of weighted residuals (WRES) versus predicted concentrations (PRED), from the base model to the final model.
The last phase of PPK analysis was validation of the final model. The validation set consisted of data obtained from new group of patients with similar characteristics as patients from index set and was used to estimate performances of the final model. Mean prediction error (MPE) and root mean squared error (RMSE) were used to evaluate bias and precision as suggested by Sheiner and Beal.Citation27
Results
The prospective pharmacokinetic study included 95 patients, 64 men and 31 women. Demographic and clinical data of the patients are shown in . Subjects were divided into two groups of similar age (building group 42.97 vs. 43.53 in validation group) and body weight (building group 75.33 vs. 75.16 in validation group). One of these groups served for construction of population models and another for its validation. After all necessary data were collected, population pharmacokinetic analysis was performed by the subroutine ADVAN4 TRANS3 of the NONMEM software. A first result of our investigation was the base model with mean population values of pharmacokinetic parameters. Minimum objective function was 307.523. Moreover, the results of the estimates of the variance have shown that the exponential error model described interindividual variability of drug clearance and residual variability much better than an additive error model in the target population.
Table 1. Characteristics of study patients.
Second phase was characterized by several univariate successive models obtained by examination of influence of every mentioned covariate. Addition of MPA total daily dose, age, donor gender, serum albumin, creatinine clearance, time after transplantation, donor status and co-medication with nifedipine and tacrolimus in the base model resulted in statistically significant reduction in MOF. All other covariates (total body weight, time after transplantation, gender, aspartate and alanine transaminase, co-medication with omeprazole and cyclosporine and prednisolone dose) in this process were excluded. The most important univariate successive models in building process are shown in . Also, all significant covariate of the full model could be seen. The value of MOF in the full model was 261.383.
Table 2. The value of MOF in the base model, univariate models and the full model.
The final population pharmacokinetics model of MPA clearance was derived by the process of backward deletion covariate from the full model. The total daily dose of the drug, age and co-medication with nifedipine met the necessary statistical requirements. The equation for the final model which describes value of population clearance is as follows:
The estimated values of population pharmacokinetic parameters in this model for CL of MPA are shown in . The interindividual variability of CL/F decreased from 48.3% to 25.1%.
Table 3. Parameter estimates for the final model.
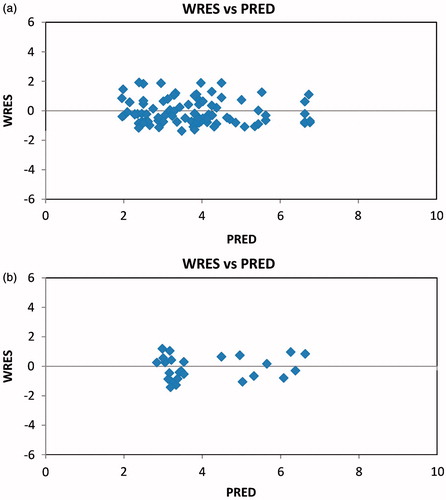
and show scatter-plots of predicted values (PRED) versus observed concentrations (DV) and weighted residuals (WRES) versus predicted concentrations (PRED) in the final model of index and validation set.
Figure 1. (a) Scatter-plots of predicted concentrations (PRED) and individual predicted concentrations (IPRED) versus observed concentrations (DV) for mycophenolic acid by the final model of index set. (b) Scatter-plots of PRED and IPRED versus DV for mycophenolic acid by the final model of validation set.
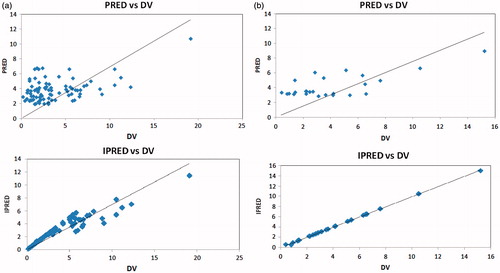
Figure 2. (a) Scatter-plot of weighted residuals (WRES) versus predicted concentrations (PRED) for mycophenolic acid by the final model of index set. (b) Scatter-plot of WRES versus PRED for mycophenolic acid by the final model of validation set.
The process of validation of the final regression model was performed on the validation set which consisted of 25 patients whose mean values of total body weight and age were 75.16 kg and 43.53 years, respectively. Calculated prediction errors of validation set to assess bias and precision are given in .
Table 4. Prediction errors of final model established in validation set.
Discussion
The pharmacokinetics of MPA exhibit considerable inter- and intra-individual variability. This study focused on developing a clinically applicable population pharmacokinetic model for MPA to quantify inter- and intra-individual variability. Also, we tried to analyze relationships between pharmacokinetic parameters, patient demographics and biochemical factors.
During population modeling, the full model showed that clearance of the MPA was significantly influenced by age, total daily dose of MPA, creatinine clearance, albumin level, status and gender of a donor and the nifedipine and tacrolimus co-therapy. In the final model, clearance of MPA was reported to be significantly influenced by age, total daily dose of MPA and the nifedipine co-therapy.
Age of the patients showed a significant effect on the clearance of MPA in our final model, which was confirmed by another research.Citation28 Some investigators demonstrate the influence of donor age on the quality of the graft at the implantation, but as well in the post-transplantation period for histological and functional evolution of the graft.Citation29 It might be the subject of further research.
Mycophenolate mofetil, a 1.5 g twice-daily starting dose, rather than a 1 g twice-daily starting dose of MMF is more likely to achieve the minimum target MPA exposure in adult transplant recipients receiving concomitant cyclosporine A therapy. Over the period between the first weeks of dosing and 1–6 months after renal transplantation, at least a 30–50% increase in MPA dose-normalized AUC has been reported.Citation8,Citation30,Citation31 This increase in drug exposure is the result of decreasing MPA apparent clearance over time and is likely to be the result of a number of factors including increasing serum albumin and hemoglobin levels, improving renal function and gradual tapering of cyclosporine, and possibly of corticosteroid dosage with increased time after transplantation.Citation22
Epidemiologic studies indicate that 50–90% of kidney transplant recipients either have hypertension (defined as blood pressure higher than 140/90 mmHg) or are on antihypertensive medications.Citation32,Citation33 Calcium-channel blockers (CCBs) have properties that make them particularly suited in the treatment of post-transplant hypertension. In 32.9% of our patients, antihypertensive therapy included nifedipine. It greatly affects the clearance of MPA due to possible inhibitory effect of nifedipine on the p-glycoprotein.Citation34 Also, nifedipine and other CCBs counteract the systemic and afferent arteriolar vasoconstriction induced by CNI, increase renal blood flow and increase glomerular filtration through an increase in the glomerular hydrostatic pressure and filtration fraction.Citation35–37 Experimental data indicate that CCBs can modulate the immune system by affecting calcium influx into T lymphocytes, limiting their activation. Some of these agents have been associated with lower risk of rejection and delayed graft function.Citation29,Citation38,Citation39
MPA binds to serum albumin and the binding is highly dependent on the concentration of albumin. A decrease in free MPA leads to a temporary decrease in its clearance, resulting in a relatively higher AUC of total MPA.Citation28 Albumin concentrations less than 3.1 g/dL have been associated with significantly higher unbound MPA concentrations in renal transplantation. Elevated unbound MPA exposure has been reported in pediatric renal transplant recipients with decreased serum albumin levels.Citation40–42 We found no impact of serum albumin on clearance of MPA, which may be explained by the fact that patients included in the study did not have hypoalbuminemia, 42.11 (range 36–49), for patients in the building model and 42.36 (range 37–49), for patients in the validation model. Also, the severity of disease or clinical status, or the impact of co-administered medications on albumin binding may explain the lack of correlation between MPA exposure and albumin in our study.
Concomitant immunosuppressive medication has a differential effect on MPA CL/F. In previous study, CL/F was 33% higher in cyclosporine A–MPA-treated patients than in tacrolimus–MPA-treated patients.Citation18 Cyclosporine A is believed to decrease MPA exposure.Citation43,Citation44 A logical explanation for this correlation may be the inhibitory effect of cyclosporin A on the enterohepatic recirculation of MPA.Citation43 Some investigators have recommended using a 50% lower dose of MMF/EC-MPS in combination with tacrolimus than of cyclosporine A.Citation30,Citation44 Tacrolimus dose demonstrated statistical significance in our full model, but in the final population model other factors showed higher impact. The observed differences with the present study may be explained by the lower frequency of cyclosporine A in the treatment of our patients.
In conclusion, clearance of MPA was estimated by using population pharmacokinetic modeling. Besides total daily dose of MPA, age and the nifedipine co-therapy seemed to be important covariates influencing MPA clearance in renal transplant recipients.
Declaration of interest
The results presented in this paper have not been published previously in whole or part. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. The financial support of this work by Ministry of Education and Science of the Republic of Serbia (Grant No. 41018 and 172044) is gratefully acknowledged.
References
- Bunnapradist S, Sampaio MS, Wilkinson AH, et al. Changes in the small bowel of symptomatic kidney transplant recipients converted from mycophenolate mofetil to enteric-coated mycophenolate sodium. Am J Nephrol. 2014;40:184–190
- Goubella A, Broeders N, Racapé J, et al. Patient and graft outcome in current era of immunosuppression: A single centre pilot study. Acta Clin Belg. 2014. [Epub ahead of print]. doi:https://doi.org/http://dx.doi.org/10.1179/2295333714Y.0000000078
- Langone A, Shihab F, Pankewycz O, et al. Long-term dosing patterns of enteric-coated mycophenolate sodium or mycophenolate mofetil with tacrolimus after renal transplantation. Clin Transplant. 2014;28:961–967
- The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. Transplantation. 1996;61:1029–1037
- Kaminska J, Glyda M, Sobiak J, Chrzanowska M. Pharmacokinetics of mycophenolic acid and its phenyl glucuronide metabolite in kidney transplant percipients with renal impairment. Arch Med Sci. 2012;8:88–96
- Abd Rahman AN, Tett SE, Staatz CE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in patients with autoimmune disease. Clin Pharmacokinet. 2013;52:303–331
- Mamelok R. From mechanisms to long-term benefits. Transplantation. 2005;79:43–44
- Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet. 2007;46:13–58
- Jeong H, Kaplan B. Therapeutic monitoring of mycophenolate mofetil. J Am Soc Nephrol. 2007;2:184–191
- Shah T, Tellez-Corrales E, Yang JW, et al. The pharmacokinetics of enteric-coated mycophenolate sodium and its gastrointestinal side effects in de novo renal transplant recipients of Hispanic ethnicity. Ther Drug Monit. 2011;33:45–49
- Arns W. Noninfectious gastrointestinal (GI) complications of mycophenolic acid therapy: A consequence of local GI toxicity? Transplant Proc. 2007;39:88–93
- Kiberd BA, Lawen J, Fraser AD, Keough-Ryan T, Belitsky P. Early adequate mycophenolic acid exposure is associated with less rejection in kidney transplantation. Am J Transplant. 2004;4:1079–1083
- Chan L, Mulgaonkar S, Walker R, Arns W, Ambühl P, Schiavelli R. Patient-reported gastrointestinal symptom burden and health-related quality of life following conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium. Transplantation. 2006;81:1290–1297
- Arns W, Breuer S, Choudhury S, et al. Enteric-coated mycophenolate sodium delivers bioequivalent MPA exposure compared with mycophenolate mofetil. Clin Transplant. 2005;19:199–206
- Langone AJ, Chan L, Bolin P, Cooper M. Enteric-coated mycophenolate sodium versus mycophenolate mofetil in renal transplant recipients experiencing gastrointestinal intolerance: A multicenter, double-blind, randomized study. Transplantation. 2011;91:470–478
- Reyes H, Hernández AM, Valverde S, et al. Efficacy and safety of conversion of mycophenolate mofetil to enteric-coated mycophenolate sodium in Mexican renal transplant children. Pediatr Transplant. 2010;14:746–752
- Musuamba FT, Mourad M, Haufroid V, et al. A simultaneous d-optimal designed study for population pharmacokinetic analyses of mycophenolic acid and tacrolimus early after renal transplantation. J Clin Pharmacol. 2012;52:1833–1843
- Zhao W, Fakhoury M, Deschenes G, et al. Population pharmacokinetics and pharmacogenetics of mycophenolic acid following administration of mycophenolate mofetil in de novo pediatric renal-transplant patients. J Clin Pharmacol. 2010;50:1280–1291
- de Winter BC, Mathot RA, van Hest RM, van Gelder T. Therapeutic drug monitoring of mycophenolic acid: Does it improve patient outcome? Expert Opin Drug Metab Toxicol. 2007;3:251–261
- Hummel M, Yonan N, Ross H, et al. Pharmacokinetics and variability of mycophenolic acid from enteric-coated mycophenolate sodium compared with mycophenolate mofetil in de novo heart transplant recipients. Clin Transplant. 2007;21:18–23
- Hohage H, Zeh M, Heck M, Gerhardt UW, Welling U, Suwelack BM. Differential effects of cyclosporine and tacrolimus on mycophenolate pharmacokinetics in patients with impaired kidney function. Transplant Proc. 2005;37:1748–1750
- Tett SE, Saint-Marcoux F, Staatz CE, et al. Mycophenolate, clinical pharmacokinetics, formulations, and methods for assessing drug exposure. Transplant Rev. 2011;25:47–57
- Li H, Mager DE, Sandmaier BM, Maloney DG, Bemer MJ, McCune JS. Population pharmacokinetics and dose optimization of mycophenolic acid in HCT recipients receiving oral mycophenolate mofetil. J Clin Pharmacol. 2013;53:393–402
- Cockroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41
- Beal SL. NONMEM Users Guides. California: NONMEM Project Group, University of San Francisko; 2006
- Milovanovic JR, Jankovic SM. Factors influencing carbamazepine PK in children and adults: Population PK-analysis. Int J Clin Pharmacol Ther. 2011;49:428–436
- Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9:503–512
- Zhou PJ, Xu D, Yu ZC, Wang XH, Shao K, Zhao JP. Pharmacokinetics of mycophenolic acid and estimation of exposure using multiple linear regression equations in Chinese renal allograft recipients. Clin Pharmacokinet. 2007;46:389–401
- Naesens M, Lerut E, de Jonge H, Van Damme B, Vanrenterghem Y, Kuypers DR. Donor age and renal p-glycoprotein expression associate with chronic histological damage in renal allografts. J Am Soc Nephrol. 2009;20:2468–2480
- Staatz CE, Duffull SB, Kiberd B Fraser AD, Tett SE. Population pharmacokinetics of mycophenolic acid during the first week after renal transplantation. Eur J Clin Pharmacol. 2005;61:507–516
- van Hest RM, Mathot RA, Pescovitz MD, Gordon R, Mamelok RD, van Gelder T. Explaining variability in mycophenolic acid exposure to optimize mycophenolate mofetil dosing: A population pharmacokinetic meta-analysis of mycophenolic acid in renal transplant recipients. J Am Soc Nephrol. 2006;17:871–880
- Kasiske BL, Anjum S, Shah R, et al. Hypertension after kidney transplantation. Am J Kidney Dis. 2004;43:1071–1081
- Ojo AO. Cardiovascular complications after renal transplantation and their prevention. Transplantation. 2006;82:603–611
- Choi JS, Choi I, Choi DH. Effects of nifedipine on the pharmacokinetics of repaglinide in rats: Possible role of CYP3A4 and P-glycoprotein inhibition by nifedipine. Pharmacol Rep. 2013;65:1422–1430
- Cross NB, Webster AC, Masson P, O'connell PJ, Craig JC. Antihypertensives for kidney transplant recipients: Systematic review and meta-analysis of randomized controlled trials. Transplantation. 2009;88:7–18
- Inigo P, Campistol JM, Lario S, et al. Effects of losartan and amlodipine on intrarenal hemodynamics and TGF-{beta}1 plasma levels in a crossover trial in renal transplant recipients. J Am Soc Nephrol. 2001;12:822–827
- Madsen JK, Sorensen SS, Hansen HE, Pedersen EB. The effect of felodipine on renal function and blood pressure in cyclosporin-treated renal transplant recipients during the first three months after transplantation. Nephrol Dial Transplant. 1998;13:2327–2334
- Vethe NT, Midtvedt K, Asberg A, Amundsen R, Bergan S. Drug interactions and immunosuppression in organ transplant recipients. Tidsskr Nor Laegeforen. 2011;131:2000–2003
- Boom H, Mallat MJ, de Fijter JW, Paul LC, Bruijn JA, van Es LA. Calcium levels as a risk factor for delayed graft function. Transplantation. 2004;77:868–873
- Kim H, Long-Boyle J, Rydholm N, et al. Population pharmacokinetics of unbound mycophenolic acid in pediatric and young adult patients undergoing allogeneic hematopoietic cell transplantation. J Clin Pharmacol. 2012;52:1665–1675
- van Hest RM, van Gelder T, Vulto AG, Shaw LM, Mathot RA. Pharmacokinetic modelling of the plasma protein binding of mycophenolic acid in renal transplant recipients. Clin Pharmacokinet. 2009;48:463–476
- de Winter BC, van Gelder T, Sombogaard F, Shaw LM, van Hest RM, Mathot RA. Pharmacokinetic role of protein binding of mycophenolic acid and its glucuronide metabolite in renal transplant recipients. J Pharmacokinet Pharmacodyn. 2009;36:541–564
- Van Hest RM, van Gelder T, Vulto AG, Mathot RA. Population pharmacokinetics of mycophenolic acid in renal transplant recipients. Clin Pharmacokinet. 2005;44:1083–1096
- Filler G, Zimmering M, Mai I. Pharmacokinetics of mycophenolate mofetil are influenced by concomitant immunosuppression. Pediatr Nephrol. 2000;14:100–104
