Abstract
Diabetic nephropathy (DN) is a serious complication and it leads to kidney failure. The endothelial nitric oxide synthases (eNOS) seems to be involved in the development and progression of DN. The Puerarin is a well-known Chinese traditional formula, which is widely used in clinical practice for the treatment of kidney disease. The present study was designed to investigate the renal protective effects of Puerarin on streptozotocin (STZ)-induced diabetic rats. Thirty Sprague–Dawley (SD) male rats were divided into three groups at random. The diabetic group and the Puerarin-treated group were intraperitoneally injected with STZ 65 mg/kg and the Puerarin-treated rats were intraperitoneally injected Puerarin 100 mg/kg/day for 4 weeks. The results showed the Puerarin could improve body weight, blood sugar, BUN and SCr levels, and reduce ultrastructural changes of kidney in diabetic rats. It also attenuated eNOS expression in glomerular endothelial cells and tubular cells of diabetic rats with Puerarin treatment (p < 0.05). The Puerarin had significant renal-protective effects for the diabetic nephropathy, possibly through regulating eNOS expression, and it may be used as a potential therapeutic reagent.
Introduction
Diabetes becomes a worldwide epidemic and more and more diabetic patients face the problems of morbidity and mortality due to chronic complications.Citation1,Citation2 There were around 177 million people with diabetes mellitus worldwide, and it has been estimated to be increased to 360 million by 2030. Given that about 20–30% of these people develop diabetic nephropathy.Citation3 Diabetes becomes a major cause of end-stage renal disease (ESRD) in several developed countries, including the USA, England and Australia.Citation4 During early stages of diabetic nephropathy, functional and structural abnormalities of glomerular capillaries occur.Citation5 The nitric oxide (NO) is believed to be involved in the early and late alterations of glomerular hemodynamics due to diabetes.Citation6–8 There are at least three NO-synthases, named neuronal NOS (nNOS), endothelial NOS (eNOS) and inducible NOS (iNOS) that are present within the kidney.Citation9 Several studies have demonstrated that increased eNOS expression seems to be involved in the development and progression of DN.Citation10,Citation11
Puerarin (daidzein-8-C-glucoside) is the main isoflavone isolated from the Chinese medicinal herb Ge-gen, the root of the wild leguminous creeper Pueraria lobata (kudzu root).Citation12 It has been shown effective in the treatment of kidney diseases, such as nephrotoxicity, acute kidney injury and nephropathy.Citation13–16 The possible mechanisms by which Puerarin exerts its protective effects, at least in part, are related to its ability to increase superoxide dismutase activity, decrease the lipid peroxidation level and enhance fibrinolysis.Citation15–19 Furthermore, the underlying mechanisms by which Puerarin plays its renal protective role in the diabetic rats are unclear.
In the present study, we investigated the eNOS expression in kidney of diabetic and Puerarin-treated rats. In addition, we related renal function and eNOS immunohistochemical data and linked to the Puerarin protective effects. The results would demonstrate the relationship between decreased eNOS expression and the protection of Puerarin in the diabetic kidney.
Materials and methods
Diabetes model induction
Thirty Sprague–Dawley (SD) male rats (from Experiment Animal Center of Zhejiang University, weight 180–200 g) were divided into three groups at random (n = 10). Diabetic group and Puerarin treatment group rats were fasted for 12 h before intraperitoneally injected streptozotocin (STZ, Alexis Corporation, Switzerland) 65 mg/kg to induce diabetes; the remaining 10 control rats also were fasted for 12 h then received an injection of 0.9% saline. 48 hours after the injection of STZ, the rat with blood glucose >16 mmol/L and urine glucose > (+) was considered for the successful induction of diabetes. Puerarin treatment group rats were then intraperitoneally injected with Puerarin injection (100 mg/kg/day, Limin Pharmaceutical Corporation, Jinan Shandong, China) for 4 weeks, the rest two groups’ rats were intraperitoneally injected with saline (6 mL/kg/day). This study was approved by the Animal Care Committee of Zhejiang University and conformed to the Guide for the Care and Use of Laboratory Animals.
Blood sample and tissue collection
After 4 weeks, body weight was measured at the conclusion of the experiment. Rats were then anesthetized with a lethal dose of intraperitoneal injection of Nembutal. Blood was collected in tubes from abdominal aorta. The serum was rapidly separated from blood and processed for determination of serum creatinine (SCr) and blood urea nitrogen (BUN). Then the rat’s thoracic cavities were opened and perfused intracardially with normal saline and fixative 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The kidney of each rat was taken out and fixed in the same fixative for 12 h and then placed in 30% phosphate-buffered sucrose until the tissue sank.
H–E stains and immunohistochemistry assay
Ten-micrometer-thick sections were cut on freezing microtome through coronary planes of kidney for hematoxylin–eosin staining and immunohistochemical staining. Sections were rinsed in 0.01 M phosphate-buffered saline (PBS) and mounted onto 0.02% poly-l-lysine-coated slides. The ABC system was used with DAB as the Chromagen. Briefly, tissue sections were first washed in PBS and then incubated in 1% bovine serum albumin (BSA) for 30 min. Tissues were then incubated overnight at 4 °C in the medium of PBS with eNOS multiclonal antibody (Boster Biotechnology Company, Wuhan, China) plus 1% BSA. The dilution of the primary antibody was 1:100. The next day, the sections were incubated in a biotinylated goat-anti-rat secondary antibody (diluted to 1:200 in PBS), and subsequently in an avidin-horseradish peroxidase (HRP) solution. Immunolabeling was visualized with 0.05% DAB plus 0.3% H2O2 in PBS. The sections were then dehydrated through ethanol and xylene before using cover slips.
For immunohistochemistry, the kidney were selected on each slide, examined at ×400 magnification and analyzed with UTHSCSA ImageTool 3.0 (University of Texas Medical School, San Antonio, TX). The number and optical density of the eNOS positive cells were measured.
Statistical analyses
All statistical analyses were performed with SPSS 11.0 (Chicago, IL). Data were presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used for statistical analysis. The differences were considered statistically significant if p-value <0.05.
Results
Clinical parameters
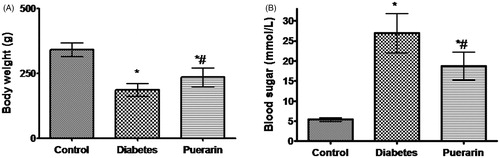
Initially, the body weight and blood glucose showed no significant differences (p > 0.05) among the three groups of rats before STZ injection. After 4 weeks, the Puerarin could improve body weight and blood glucose compared to the diabetic group. There were lower body weight and higher blood glucose level in both groups than that of the control group (p < 0.05, ).
Figure 1. Body weight and blood glucose in three groups 4 weeks after STZ injection. (A) Body weight in three groups, (B) Blood glucose in three groups. *p < 0.05 vs. control group, #p < 0.05 vs. diabetic group.
In the diabetes group, BUN and SCr were significantly increased (p < 0.05) when compared to normal rats, treatment with Puerarin attenuated BUN and SCr from the diabetic rats (p < 0.05), but BUN and SCr were still higher than that observed in the control group (p < 0.05) ().
Table 1. The BUN and SCr in the three groups of rats 4 weeks after STZ injection.
Hematoxylin and eosin staining of the kidney tissues
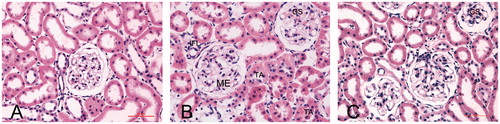
Histopathological studies of the kidneys revealed that the NC group presented kidneys with normal architecture (). We observed that diabetic glomerular lesion is characterized by mesangial expansion (ME) and global sclerosis (GS). There is tubular atrophy (TA), interstitial fibrosis (IF) and focal interstitial inflammation (IFL) (). These abnormal pathological findings of tissue injury were reduced in Puerarin-treated rats (). In more advanced stages of DN, glomerulosclerosis is evident, with a massive accumulation of ECM components in the mesangium at the expense of capillary volume. Moreover, hyaline arteriolosclerosis is often prominent ().
Figure 2. Light photomicrographs showing the kidney morphology by hematoxylin and eosin stain (400×). (A) A control rat, (B) a diabetic rat and (C) a Puerarin-treated rat. The diabetic glomerular lesion is characterized by mesangial expansion (ME), global sclerosis (GS). There is tubular atrophy (TA), interstitial fibrosis (IF) and focal interstitial inflammation (IFL).
eNOS immunohistochemistry of the kidney
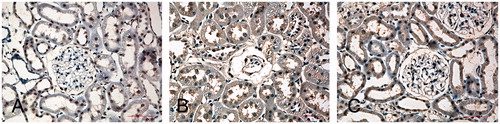
Cells that stained positive for eNOS showed buff-colored granules with DAB staining. shows light photomicrographs of eNOS immunohistochemical staining from the three groups of rats. The expression of eNOS was virtually absent in glomerular endothelial cells, but positive in some tubular cells in the kidney of normal rats (), while the diabetic kidney showed enhanced eNOS expression in glomerular endothelial cells (), which represented the major source of eNOS especially with severe glomerulosclerosis. In addition, there was also positivity for eNOS next to injured capillaries that might originate from other cells such as mesangial or invading inflammatory cells. In contrast, the tubular eNOS expression only slightly increased compared with kidneys of normal rat. Puerarin-treated animals had a significantly decreased number of eNOS positive cells (15.1 ± 3.2/mm2) and decreased optical density (108.9 ± 17.3) of the eNOS immunostained compared with the diabetic rats (21.2 ± 4.3/mm2 and 152.7 ± 20.2), while the eNOS positive cells and optical density in the control group were 5.4 ± 1.2/mm2 and 83.4 ± 8.3 (p < 0.05, ).
Figure 3. Light photomicrographs of eNOS immunohistochemical staining of the kidney (400×). (A) A control rat, (B) a diabetic rat and (C) a Puerarin-treated rat. The expression of eNOS was virtually absent in glomerular endothelial cells, but positive in some tubular cells in the kidney of normal rat (A), while the diabetic kidney showed enhanced eNOS expression in glomerular endothelial cells (B), which represented the major source of eNOS especially with severe glomerulosclerosis. In addition, there was also positivity for eNOS next to injured capillaries that might originate from other cells, such as mesangial or invading inflammatory cells. In contrast, the tubular eNOS expression only slightly increased compared with kidneys of normal rat.
Discussion
In the present study, we investigated the renal protective effects of Puerarin in rats with diabetic nephropathy. The results demonstrated that diabetes could induce mesangial expansion, global sclerosis, tubular atrophy and interstitial inflammation. All these pathological changes caused the symptoms of nephropathy. Interestingly, these abnormal pathological changes of kidney injury were reduced in Puerarin-treated rats. Furthermore, hyperglycemia could impair the kidney function as it has higher BUN and SCr levels when compared to normal rats, while the Puerarin-treated diabetic rats could improve BUN and SCr levels.
Many experimental diabetic models have demonstrated an increase of eNOS expression by immunohistochemistry and at the mRNA level in the kidney.Citation6,Citation20 It is suggested that diabetes triggers dysfunction of NO bioavailability. Increased eNOS might be a compensatory mechanism to endothelial damage in type 2 diabetes by an excess production of ROS related to ineffective NO action.Citation21 The enhanced NO production may contribute to hyperfiltration and microalbuminuria that characterizes early diabetic nephropathy.Citation22 The results of quantitative and qualitative expression patterns of the eNOS demonstrated that eNOS was virtually absent in control glomeruli but positive in some mesangial areas, glomeruli of diabetic nephropathy had an increased typical and prominent endothelial staining pattern. This pattern changed with ongoing disease and during ongoing injury, eNOS expression was mainly detected in areas of developing sclerosis.Citation23,Citation24 The present results also demonstrated an increase of eNOS in glomerular and tubular cells of diabetic kidneys. Our finding of increased glomerular eNOS expression was believed to be involved in abnormal pathological findings of kidney injury and increased SCr and BUN and contributed to the development and progression of DN, while the Puerarin treatment could improve them.
Modern pharmacology has demonstrated that Puerarin can eliminate stasis, improve micro-circulation, inhibit aldose reductase activity and decrease blood glucose.Citation25–28 Furthermore, it has been shown to have clinical applications in the treatment of coronary heart disease and diabetic nephropathy.Citation29–31 The previous study found that Puerarin may induce therapeutic angiogenesis in myocardium by inducing VEGF and eNOS expression in myocardium of rat with myocardial infarction.Citation32,Citation33 It would scavenge free radicals, lower serum cholesterol, and have antithrombotic and antiallergic activities. From the present results, we suggested that Puerarin-treated animals had a significantly decreased eNOS expression in glomerular and tubular cells under diabetes condition. It also improved abnormal pathological changes of kidney injury, SCr and BUN.
In conclusion, the present results indicated that Puerarin did not only successfully promote abnormal pathological changes of kidney in diabetic rats, but it also improved the renal function as to decrease SCr and BUN. The underlying mechanism may be that Puerarin can decrease eNOS expression in glomerular and tubular cells under diabetes condition. The mechanism of Puerarin protective effects in kidney of rat with diabetes is novel and preliminary. Thus, Puerarin treatment may represent a novel approach for improving DN and the clinical application will be further investigated.
Declaration of interest
The authors have no conflicts of interest to declare in relation to this paper.
References
- Jia W, Xu A, Chen A, Wu J, Ye J. Chronic vascular complications in diabetes. J Diabetes Res. 2013;2013:858746
- Wang F, Huang B, Li J, Liu L, Wang N. Renalase might be associated with hypertension and insulin resistance in Type 2 diabetes. Ren Fail. 2014;36(4):552–6
- Balakumar P, Bishnoi HK, Mahadevan N. Telmisartan in the management of diabetic nephropathy: A contemporary view. Curr Diabetes Rev. 2012;8(3):183–90
- Lopes AA. End-stage renal disease due to diabetes in racial/ethnic minorities and disadvantaged populations. Ethn Dis. 2009;19(1 Suppl 1):S1-47–S1-51
- Sahay M, Mahankali RK, Ismal K, Vali PS, Sahay RK, Swarnalata G. Renal histology in diabetic nephropathy: A novel perspective. Indian J Nephrol. 2014;24(4):226–231
- Dellamea BS, Leitao CB, Friedman R, Canani LH. Nitric oxide system and diabetic nephropathy. Diabetol Metab Syndr. 2014;6(1):17
- Cheng H, Wang H, Fan X, Paueksakon P, Harris RC. Improvement of endothelial nitric oxide synthase activity retards the progression of diabetic nephropathy in db/db mice. Kidney Int. 2012;82(11):1176–1183
- Zeng R, Duan L, Sun L, et al. A meta-analysis on the relationship of eNOS 4b/a polymorphism and diabetic nephropathy susceptibility. Ren Fail. 2014;36(10):1520–1535
- Morisada N, Nomura M, Nishii H, et al. Complete disruption of all nitric oxide synthase genes causes markedly accelerated renal lesion formation following unilateral ureteral obstruction in mice in vivo. J Pharmacol Sci. 2010;114(4):379–389
- Arya A, Yadav HN, Sharma PL. Involvement of vascular endothelial nitric oxide synthase in development of experimental diabetic nephropathy in rats. Mol Cell Biochem. 2011;354(1–2):57–66
- Dellamea BS, Pinto LC, Leitao CB, Santos KG, Canani LH. Endothelial nitric oxide synthase gene polymorphisms and risk of diabetic nephropathy: A systematic review and meta-analysis. BMC Med Genet. 2014;15:9
- Xu L, Zheng N, He Q, Li R, Zhang K, Liang T. Puerarin, isolated from Pueraria lobata (Willd.), protects against hepatotoxicity via specific inhibition of the TGF-beta1/Smad signaling pathway, thereby leading to anti-fibrotic effect. Phytomedicine. 2013;20(13):1172–1179
- Ma JQ, Ding J, Xiao ZH, Liu CM. Puerarin ameliorates carbon tetrachloride-induced oxidative DNA damage and inflammation in mouse kidney through ERK/Nrf2/ARE pathway. Food Chem Toxicol. 2014;71:264–271
- She S, Liu W, Li T, Hong Y. Effects of Puerarin in STZ-induced diabetic rats by oxidative stress and the TGF-beta1/Smad2 pathway. Food Funct. 2014;5(5):944–950
- Zhong Y, Zhang X, Cai X, Wang K, Chen Y, Deng Y. Puerarin attenuated early diabetic kidney injury through down-regulation of matrix metalloproteinase 9 in streptozotocin-induced diabetic rats. PLoS One. 2014;9(1):e85690
- Wang L, Lin S, Li Z, Yang D, Wang Z. Protective effects of Puerarin on experimental chronic lead nephrotoxicity in immature female rats. Hum Exp Toxicol. 2013;32(2):172–185
- Wang L, Lin S, Li Z, Yang D, Wang Z. Protective effects of Puerarin on experimental chronic lead nephrotoxicity in immature female rats. Hum Exp Toxicol. 2013;32(2):172–185
- Li Q, Xiao Y, Gong H, et al. Effect of Puerarin on the expression of extracellular matrix in rats with streptozotocin-induced diabetic nephropathy. Natl Med J India. 2009;22(1):9–12
- Ma JQ, Ding J, Xiao ZH, Liu CM. Puerarin ameliorates carbon tetrachloride-induced oxidative DNA damage and inflammation in mouse kidney through ERK/Nrf2/ARE pathway. Food Chem Toxicol. 2014;71:264–271
- Advani A, Connelly KA, Advani SL, et al. Role of the eNOS-NO system in regulating the antiproteinuric effects of VEGF receptor 2 inhibition in diabetes. Biomed Res Int. 2013;2013:201475
- Jayakumari NR, Reghuvaran AC, Rajendran RS, et al. Are nitric oxide- mediated protein modifications of functional significance in diabetic heart? ye's, -NO', wh'y-NO't? Nitric Oxide. 2014;43:35–44
- Prabhakar SS. Role of nitric oxide in diabetic nephropathy. Semin Nephrol. 2004;24(4):333–344
- Okada S, Saito M, Kazuyama E, et al. Effects of N-hexacosanol on nitric oxide synthase system in diabetic rat nephropathy. Mol Cell Biochem. 2008;315(1–2):169–177
- Komers R, Anderson S. Glomerular endothelial NOS (eNOS) expression in type 2 diabetic patients with nephropathy. Nephrol Dial Transplant. 2008;23(9):3037; author reply 3037–3038
- Liang J, Chen H, Pan W, Xu C. Puerarin inhibits caspase-3 expression in osteoblasts of diabetic rats. Mol Med Rep. 2012;5(6):1419–1422
- Wang Y, Li J, Zhuge L, Su D, Yang M, Tao S. Comparison between the efficacies of curcumin and Puerarin in C57BL/6 mice with steatohepatitis induced by a methionine- and choline-deficient diet. Exp Ther Med. 2014;7(3):663–668
- Zhu LH, Wang L, Wang D, et al. Puerarin attenuates high-glucose-and diabetes-induced vascular smooth muscle cell proliferation by blocking PKCbeta2/Rac1-dependent signaling. Free Radic Biol Med. 2009;48(4):471–482
- Zhong Y, Zhang X, Cai X, Wang K, Chen Y, Deng Y. Puerarin attenuated early diabetic kidney injury through down-regulation of matrix metalloproteinase 9 in streptozotocin-induced diabetic rats. PLoS One. 2014;9(1):e85690
- Chen R, Xue J, Xie M. Puerarin prevents isoprenaline-induced myocardial fibrosis in mice by reduction of myocardial TGF-beta1 expression. J Nutr Biochem. 2011;23(9):1080–1085
- Wu L, Qiao H, Li Y, Li L. Protective roles of Puerarin and Danshensu on acute ischemic myocardial injury in rats. Phytomedicine. 2007;14(10):652–658
- Hou Q, Ao X, Li G, Zhang Y. Puerarin combined with avandia for diabetic nephropathy. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2012;37(1):73–77
- Lu XL, Liu JX, Wu Q, et al. Protective effects of Puerarin against Ass40-induced vascular dysfunction in zebrafish and human endothelial cells. Eur J Pharmacol. 2014;732:76–85
- Zhang S, Chen S, Shen Y, et al. Puerarin induces angiogenesis in myocardium of rat with myocardial infarction. Biol Pharm Bull. 2006;29(5):945–950
