Abstract
Dexmedetomidine (dex) is a potent, highly selective and specific α2-adrenoreceptor agonist. This experimental study was designed to investigate protective and therapeutic effect of two different doses of dex, on kidney damage induced by ischemia-reperfusion (I/R) in rats. Male Sprague−Dawley rats were divided into four groups, each including 10 animals: control group, ischemia-reperfusion (I/R) group; treated groups with 10 μg/kg of dex and 100 μg/kg of dex. After removing right kidney of the rats, the left kidney has performed ischemia during 40 min and reperfusion in the following 3 h. The histopathological findings, and also tissue superoxide dismutase (SOD) and catalase (CAT) enzyme activity, malondialdehyde (MDA), glutathione (GSH), serum blood urea nitrogen (BUN), creatinine (Cre) and tumor necrosis factor-alpha (TNF-α) levels were determined. In the I/R group, compared to the control group, levels of BUN, Cre and kidney tissue MDA have increased significantly, SOD, CAT enzyme activity and glutathione levels have decreased significantly. In the dex10 group, compared to the I/R group, levels of Cre and TNF-α have decreased significantly, while the SOD activity has increased significantly. In the dex100 group, compared to the I/R group, levels of BUN, Cre have decreased significantly, while the SOD activity has increased significantly. In the I/R group, there was also extensive tubular necrosis, glomerular damage in the histological evaluation. Dex ameliorated these histological damages in different amounts in two treatment groups. In this study, the protective effects of dex against renal I/R injury have been evaluated by two different amount of doses.
Introduction
Renal ischemia-reperfusion (I/R) injury is associated with increased mortality and morbidity rates caused by acute renal failure (ARF). Different clinical–surgical procedures, such as kidney transplantation, bypass processes, renal angioplasty, and clamping of the renal pedicle or the aorta above the renal arteries are frequent urological or vascular events and require temporary interruption or reduction of the renal perfusion flux.Citation1 Although the return of blood flow to ischemic tissue can result in recovery of normal functions, the tissue may also be injured paradoxically during the reperfusion.Citation2 Free radical species that are known to be greatest relevance to reperfusion injury are the oxygen-centered free radicals, which include the hydroxyl radical (OH−), hydrogen peroxide (H2O2) and the superoxide radical (O2−). The post-ischemic endothelium is the main source of the O2− (intracellular sources are mitochondria) that, either alone or in interest with other factors, results in important changes within the endothelium.Citation3 The organ dysfunction that accompanies this condition is generally associated with increased microvascular permeability, interstitial edema, reduced vasoregulation, inflammatory cell infiltration, and parenchymal cell dysfunction and necrosis.Citation4
Dexmedetomidine (dex) is a potent, highly selective and specific α2-adrenoreceptor agonist that has sedative and analgesic effects. Dexmedetomidine was approved in the USA in 1999 to be used as sedation and analgesia in the intensive care unit.Citation5 The hemodynamic effects of dex result from peripheral and central mechanism. The net effect of dex action is a significant reduction in circulating catecholamine with a mild decrease in blood pressure and a modest reduction in heart rate. Stimulation of α2-adrenoreceptors in the kidneys results in diuresis and natriuresis possibly through an ability to reduce efferent sympathetic outflow of the renal nerve. In addition, dexmedetomidine has shown to decrease the secretion of vasopressin and to antagonize its effect on renal tubules. α2-adrenoreceptor agonists are also expected to increase the release of atrial natriuretic peptide resulting in natriuresis.Citation6 The purpose of this experimental study was to evaluate the biochemical and histologic effects of dexmedetomidine on kidney IR injury in rats.
Materials and methods
Animals and experimental protocol
The experimental protocol in our study was approved by the Ethical Committee on Animal Research of Inonu University. Forty male Sprague–Dawley rats, weighing 320–370 g, were brought from Inonu University Laboratory Animals Research Center and kept in controlled room on a standard commercial pellet diet and water ad libitum with a temperature of (21 ± 2 °C), humidity (60 ± 5%) and light (12:12-h light and dark cycle). The rats were randomly divided into four groups. Rats were anesthetized with urethane (1.2 g/kg) intraperitoneally (ip) before surgical operation. Control group rats (n = 10), apply to any surgical procedure, were sacrificed by taking blood from their hearts. In I/R untreated group rats (n = 10), abdomen was dissected, the right kidney was harvested. Then, the left renal artery and vein were occluded together by a clamp for 40 min followed by reperfusion for 3 h. For the dex10 group rats (n = 10), the same surgical procedure as in the I/R group was performed. 10 μg/kg of dexmedetomidine hydrochloride, ip (Precedex 100 µg/2 ml, Abbott, Abbott Laboratory, North Chicago, IL) was applied at the starting time of ischemia. For the dex100 group (n = 10), the same surgical procedure as in the I/R group was performed. 100 μg/kg of dexmedetomidine (Dexmedetomidine hydrochloride), ip was applied at the starting time of ischemia.
After these operations, the experimental animals were sacrificed by taking blood from their hearts and the left kidney were quickly removed, de-capsulated and divided equally into two longitudinal sections. One of these sections was placed in formaldehyde solution for routine histopathological examination by the light microscopy. The other half of the kidney was stored in deep freezer at −80 °C until assayed for MDA, SOD, CAT and GSH. The blood obtained was extracted to evaluate serum levels of BUN and Cre by using an Olympus Autoanalyzer (Olympus Instruments, Tokyo, Japan). TNF-α were measured by ELISA according to the commercial kits rat (Ebioscience, Ireland, UK).
Biochemical analyses
Determination of MDA
The MDA contents of the homogenates were determined spectrophotometrically by measuring the presence of thiobarbituric acid reactive substances.Citation7 The absorbance was measured by spectrophotometer (UV-1601; Shimadzu, Kyoto, Japan) at 532 and 520 nm. The results were expressed in nmol/g tissue, according to a prepared standard graph.
Determination of SOD activity
Total SOD activity was determined in accordance with the method developed by Sun et al.Citation8 The principle of the method is the inhibition of nitroblue tetrazolium (NBT) reduction by the xanthine–xanthine oxidase system as a superoxide generator. SOD activity was expressed in U/g protein.
Determination of CAT activity
Catalase activity was determined in accordance with a method developed by Aebi.Citation9 The principle of the assay is based on the determination of the rate constant (k, s−1) or the H2O2 decomposition rate at 240 nm. Results were expressed as in k/g protein.
Determination of GSH activity
Reduced GSH was determined by the spectrophotometric method, which was based on the use of Elman’s reagent.Citation10 Results were expressed as in nmol/mg tissue.
Histological evaluation
The kidney tissue was fixed in 10% formalin and was embedded in paraffin. Sections of the tissue were cut at 5 µm, mounted on slides, stained with hematoxylin–eosin (H–E). Sections were evaluated for the presence of tubular epithelial cell desquamation, interstitial hemorrhage and glomerular shrinkage. The histological slides of kidney were evaluated for semi-quantitative analysis and graded as follows: score 0 = no cortical area injury; score 1 = 0–25% of the cortical area was injured; score 2 = 25–50% of the cortical area was injured; score 3 = 50–75% of the cortical area was injured and score 4 ≥ 75% of the cortical area was injured. The sections were examined by a Leica DFC 280 light microscope (Leica Micros Imaging Solutions Ltd., Cambridge, UK).
Statistical analysis
The statistical analyses were carried out by SPSS15.0 (SPSS Inc., Chicago, IL). The quantitative data were expressed as mean ± standard deviation and median (min–max) values. The distribution of quantitative variables in each group was examined by the Kolmogorov–Smirnov test. The one-Way ANOVA analysis was used for the comparison of variables showing normal distribution; while post-hoc Tukey test was employed for multiple comparisons of the variables showing homogeneous variance and Tamhane T2 test was used for those that do not show homogeneous variance. In the comparison of the variables, not distributed normally, Kruskal–Wallis analysis and Mann–Whitney U-test with Bonferroni’s correction were used. The value of p < 0.05 was considered to be statistically significant.
Results
Effect of dexmedetomidine on I/R-induced changes in kidney tissue enzymes, GSH and lipid peroxides
As shown in , the MDA level in the I/R group was higher than that in the control group (p < 0.05). The tissue SOD, CAT enzyme activity and levels of GSH in the I/R group were lower than those in the control group (p < 0.05). The groups of dex10 and dex100 demonstrated lower GSH levels compared to the control group (p < 0.05). The increase of SOD activity in the groups of dex10 and dex100 were significant compared to those in the I/R group (p < 0.05).
Table 1. The levels of MDA‚ GSH and SOD‚ CAT enzyme activity in kidney tissue.
Effect of dexmedetomidine on serum parameters
As shown in , briefly, serum levels of BUN and Cre were significantly higher in the I/R group, dex10 and dex100 groups compared to the control group (p < 0.05). In group dex100, the elevated BUN levels were significantly lower than those in the I/R group (p < 0.05). In groups of dex10 and dex100, the elevated Cre levels were significantly lower than those in the I/R group (p < 0.05). The TNF-α levels of I/R, dex10 and dex100 groups did not significantly change compared to the TNF-α levels of the control group. In dex10 group, TNF-α levels were significantly lower than those in the I/R group (p < 0.05).
Table 2. The serum levels of BUN‚ Cr, TNF-α.
Histological examination
The histologic injury score in the I/R group were higher than in the control group (p < 0.05). However, the histologic injury scores were lower in groups dex10 and dex100 than those in the I/R group (p < 0.05). There was no significant difference in the histologic injury scores between dex10 and dex100 groups (). The kidneys of the control group have shown normal histologic features (). However, in the I/R group, an extensive cortical damage was observed. The affected glomeruli have shown hypocellularity and shrinkage (). Most of the cortical tubules have shown morphologic changes, such as desquamation (). Furthermore, interstitial hemorrhage was observed in the I/R group (). The dex10 group has not completely ameliorated the lesion and degenerative changes, such as hemorrhage were still present (). On the other hand, applying 100 µg was effective than the application of 10 μg. The histological appearance in the dex100 group was similar to the control group except slight hemorrhagia ().
Figure 1. (A) Histopathological Score. (B) Control group: glomeruli (g) and tubules (t) appear normal. *p < 0.05 vs. Group control, **p < 0.05 vs. Group I/R. H–E;X66.
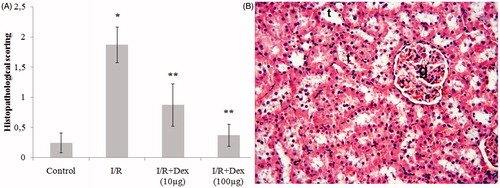
Figure 2. I/R Group: (A) Glomeruli (g) are hypocellularity and shrinkage. Interstitial hemorrhage is evident (asterisks). (B) Desquamated epithelial cells are visible in the lumens of tubules (arrows). H–E;X66.
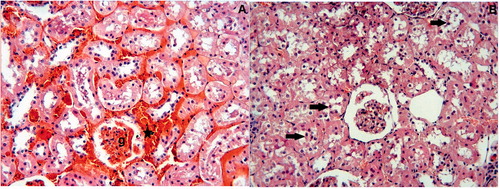
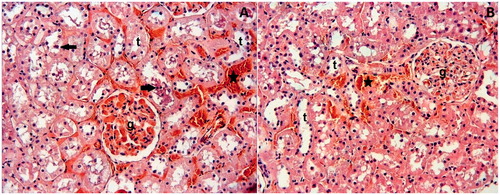
Figure 3. (A) Dex10 Group: While glomeruli (g) appear normal, tubules (t) and interstitial area show slight degenerative changes, such as epithelial desquamation (arrows) and interstitial hemorrhage (asterisks). (B) Dex100 Group: The glomeruli and tubules appear nearly normal. Slight interstitial hemorrhage (asterisks) is present. H–E; X66.
Discussion
In this study, it has been determined that I/R applied to the kidney reduces the antioxidant enzyme activity, increases the lipid peroxidation indicator MDA, causes increase in the level of BUN, Cre as a result of the deterioration in renal functioning. During I/R, it has been concluded that the practice of dex, which is α2 adrenergic receptor agonist, results in an increase in the antioxidant enzyme activity, and a decrease in the level of BUN, Cre, TNF-α and in the histological examination, it plays an important role to reduce the damage given by I/R.
The reperfusion of an ischemic organ, often results in severe tissue damages; but this situation is mostly caused by re-oxygenation rather than the ischemic period. In several studies, after re-oxygenation despite the increase in the amount of reactive oxygen species (ROS), antioxidant defense system may lead to inability to tissue damage.Citation11
Reperfusion of ischemic tissues further increases inflammatory reactions. This case occurs due to an increase in the release of free radicals and accumulation of inflammatory mediators and immune cells giving damage.Citation12 In many tissues, an important feature of I/R associated inflammatory response is induction of chemokines. ROS triggers the cascade of cytokines and chemokines through NF-kB (nuclear factor kappa B) activation. The release of proinflammatory cytokines, such as TNF-α and IL-1β (interleukin beta) induces chemokine synthesis in ischemic tissues.Citation13,Citation14 During the TNF-α renal I/R, the macrophages, leukocytes and proinflammatory released from renal tubular cells are cytokines.Citation15
According to a study carried out by Gu et al.Citation16 in mice, it has been reported that after kidney I/R, dex reduces the levels of myeloperoxidase (MPO) in the lung tissue, inter-cellular adhesion molecule (ICAM-1) and TNF-α mRNA. In another study, it has been determined that dex reduces the level of MDA and increases the level of GSH after hepatic I/R. In the same study, it has been reported that SOD, CAT and GSH peroxidase (GSH-Px), enzyme activation have been increased.Citation17
Sugita et al.Citation18 have reported that the dex infusion reduces the renal dysfunction developed by I/R during bilateral renal I/R, and also reduces renal parenchyma interleukin-6 (IL-6), inducible nitric oxide synthase (iNOS) and ICAM-1 mRNA expression. In another study, it has been observed that the dex application reduces kidney caspase 3, plasma ICAM-1 and monocyte chemo attractant protein-1 (MCP-1) levels; and these effects have been inhibited by atipamezole, which is α-2 antagonist.Citation19 These results suggest that the dex causes these effects through α-2 adrenergic receptors.
Acute renal failure is a case, in which sudden drops can be observed in the glomerular filtration rate and accumulation of waste products, such as BUN, Cre can occur.Citation20 The rise in the levels of BUN and Cre is an indication of the deterioration in renal function.Citation21
Si et al.Citation19 have reported that the dex causes increases in the serum BUN and Cre levels and reduce the damage in kidney tissue after renal I/R. Kocoglu et al.Citation22 have stated that the dex reduces the tubular damage after kidney I/R.
In our study, in the I/R group, the levels of BUN, Cre and MDA have been increased and the widespread cortical damage, interstitial hemorrhage in the histological examination and the levels of GSH, enzyme activities of SOD and CAT have been decreased, compared to the control group. These findings show that there are some reductions in the renal function and oxidative damages occur in the kidney tissue due to I/R damage. In a comparison of the I/R group with other treated groups, we have observed that the levels of Cre and TNF-α decrease, the SOD enzyme activity increases and there are some reductions in the tubular damage in the group dex10. In the dex100 group, the level of Cre and BUN decreases, the SOD enzyme activity increases and some similar results have been found with the control group in histological examination. These results suggest that applying 100 µg/kg of dex instead of 10 µg/kg of dex is more effective in terms of healing the kidney I/R damages. Besides our findings, the reducing effect of Dex in terms of anesthetic and analgesic needs suggests that it can be used during surgical procedures.
In this experimental study, we have found that the 10 μg/kg and 100 μg/kg doses of dex have a protective effect in the kidney I/R damages, which is consistent with the other studies in the literature. Unlike other studies in the literature, we have investigated the effect of dex on the oxidative stress occured during renal I/R and we have determined that it is protective against the stress occurred during I/R. There is a need of further studies investigating the clinical use of dex for I/R damages.
Declaration of interest
This study was supported by Inonu University Department of Scientific Research Projects (Project no: 2011/59). No competing interests declared.
References
- Sehirli AO, Sener G, Ercan F. Protective effects of pycnogenol against ischemia-reperfusion induced oxidative renal injury in rats. Ren Fail. 2009;31:690–697
- Koo DD, Welsh KI, West NE, et al. Endothelial cell protection against ischemia/reperfusion injury by lecithinized superoxide dismutase. Kidney Int. 2001;60:786–796
- Anaya-Prado R, Toledo-Pereyra LH. The molecular events underlying ischemia-reperfusion injury. Transpl Proc. 2002;34:2518–2519
- Granger DN, Korthuis RJ. Physiological mechanisms of postischemic tissue injury. Annu Rev Physiol. 1995;57:311–332
- Ma D, Rajakumaraswamy N, Maze M. α2-Adrenoceptor agonists: Shedding light on neuroprotection? Br Med Bull. 2005;71:77–92
- Arcangeli A, Alo CD, Gaspari R. Dexmedetomidine use in general anaesthesia. Curr Drug Targets. 2009;10:687–695
- Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;34:271–278
- Sun Y, Oberley L, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500
- Aebi H. Catalase methods of enzymatic analysis. In: Bergmeyer HU, ed. Methods of Enzymatic Analysis. New York: Academic Press; 1974;673–677
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;8:70–77
- Das DK, Maulik N. Antioxidant effectiveness in ischemia-reperfusion tissue injury. Methods Enzymol. 1994;233:601–610
- Ysebaert DK, De Greef KE, Vercauteren SR, et al. Identification and kinetics of leukocytes after severe ischemia/reperfusion renal injury. Nephrol Dial Transplant. 2000;15:1562–1574
- Frangogiannis NG. Chemokines in ischemia and reperfusion. Thromb Hemost. 2007;97:738–747
- Thurman JM. Triggers of Inflammation after renal ischemia/reperfusion. Clin Immunol. 2007;123:7–13
- Suyani E, Derici UB, Sahin T, et al. Effects of everolimus on cytokines, oxidative stress and renal histology in ischemia-reperfusion injury of the kidney. Ren Fail. 2009;31:698–703
- Gu J, Chen J, Xia P, Tao G, Zhao H, Ma D. Dexmedetomidine attenuates remote lung injury induced by renal ischemia-reperfusion in mice. Acta Anaesthesiol Scand. 2011;55:1272–1278
- Sahin T, Begeç Z, Toprak Hİ, et al. The effects of dexmedetomidine on liver ischemia-reperfusion injury in rats. J Surg Res. 2013;183:385–390
- Sugita S, Okabe T, Sakamoto A. Continuous infusion of dexmedetomidine improves renal ischemia-reperfusion injury in rat kidney. J Nippon Med Sch. 2013;80:131–139
- Si Y, Bao H, Han L, et al. Dexmedetomidine protect against renal ischemia and reperfusion injury by inhibiting the JAK/STAT signaling activation. J Transl Med. 2013;141:9–11
- Lameire NH, Vanholder R. Pathophysiology of ischemic acute renal failure. Best Pract Res Clin Anaesthesiol. 2004;18:21–36
- Edelstein CL. Biomarkers of acute kidney injury. Adv Chronic Kidney Dis. 2008;15: 222–234
- Kocoglu H, Ozturk H, Ozturk H, Yilmaz F, Gulcu N. Effect of dexmedetomidine on ischemia-reperfusion injury in rat kidney: A histopathologic study. Ren Fail. 2009;31:70–74
